SUMMARY
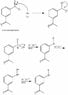
The reaction mechanism for converting m-nitroacetophenone to m-aminoacetophenone involves a two-step process: first, the reduction of m-nitroacetophenone using tin and hydrochloric acid (HCl), followed by the treatment with sodium hydroxide (NaOH) to yield m-aminoacetophenone. This mechanism is essential for understanding the reduction and subsequent amination processes in organic chemistry. The discussion highlights the urgency of clarifying the mechanism for a lab report due the next day, indicating its relevance in academic settings.
PREREQUISITES
- Understanding of organic chemistry reaction mechanisms
- Familiarity with reduction reactions using tin and HCl
- Knowledge of amination processes involving sodium hydroxide
- Basic laboratory skills for conducting chemical reactions
NEXT STEPS
- Research the detailed mechanism of reduction reactions involving tin and HCl
- Study the amination process of aromatic compounds using sodium hydroxide
- Explore the role of nitro groups in organic synthesis
- Review similar reaction mechanisms in organic chemistry textbooks
USEFUL FOR
Chemistry students, organic chemists, and laboratory technicians involved in synthesis and reaction mechanism analysis.