- #1
Aleoa
- 128
- 5
In the first volume of his lectures (cap 6-4) Feynman presents Maxwell's distribution of velocities of the molecules in a gas.
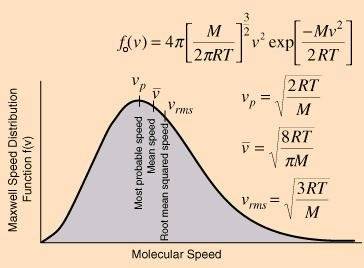 And, referring to the PDF graph he says:
And, referring to the PDF graph he says:
"If we consider the molecules in a typical container (with a volume of, say, one
liter), then there are a very large number N of molecules present (N ≈ [itex]10^{22}[/itex]).
Since p(v) ∆v is the probability that one molecule will have its velocity in ∆v,
by our definition of probability we mean that the expected number <∆N> to be
found with a velocity in the interval ∆v is given by: [itex]<\Delta N>=Np(v)\Delta v[/itex].
[...]
Since with a gas we are usually dealing with large numbers of molecules,
we expect the deviations from the expected numbers to be small (like 1/√N), so
we often neglect to say the “expected” number"
I have not understand this part, how it's possible to derive this 1/√N deviation ( or a value the gives the idea of what Feynman is saying) ?
Thanks for your support
"If we consider the molecules in a typical container (with a volume of, say, one
liter), then there are a very large number N of molecules present (N ≈ [itex]10^{22}[/itex]).
Since p(v) ∆v is the probability that one molecule will have its velocity in ∆v,
by our definition of probability we mean that the expected number <∆N> to be
found with a velocity in the interval ∆v is given by: [itex]<\Delta N>=Np(v)\Delta v[/itex].
[...]
Since with a gas we are usually dealing with large numbers of molecules,
we expect the deviations from the expected numbers to be small (like 1/√N), so
we often neglect to say the “expected” number"
I have not understand this part, how it's possible to derive this 1/√N deviation ( or a value the gives the idea of what Feynman is saying) ?
Thanks for your support
