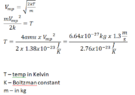greg997
- 105
- 2
- Homework Statement
- Finding Temperatur T and Pressure of gas in container given N= number of atoms of hellium and distribution V most probable.
- Relevant Equations
- Vmp= sqrt(2kT/m)
Can someone explain to me what I am doing wrong? Trying to calculate the Temperature T using this formula
I am trying to find T using most probable speed of atoms formula.
T = (V^2) m/( 2k) I am getting riduculous T like 20x10^27. Why?
If number of atoms N= 2x10^24 how do i get mass m? each hellium atom has 4 amu.
What i get is this : 2x10^24 x 4 = 8x10^24 amu
then 8x10^24 x 1.66x 10^-27 = 13.28x10^-3= m
is that correct?
I am trying to find T using most probable speed of atoms formula.
T = (V^2) m/( 2k) I am getting riduculous T like 20x10^27. Why?
If number of atoms N= 2x10^24 how do i get mass m? each hellium atom has 4 amu.
What i get is this : 2x10^24 x 4 = 8x10^24 amu
then 8x10^24 x 1.66x 10^-27 = 13.28x10^-3= m
is that correct?