- #1
asifion
- 18
- 0
- Homework Statement
- You are watching a science fiction movie in which the hero shrinks down to the size of an atom and fights villains while jumping from air molecule to air molecule. In one scene, the hero's molecule is about to crash head-on into the molecule on which a villain is riding. The villain's molecule is initially 50 molecular radii away and, in the movie, it takes 3.0 s for the molecules to collide. Estimate the air temperature required for this to be possible. Assume the molecules are nitrogen molecules, each traveling at the rms speed.
- Relevant Equations
- rms speed=√((3k_B * T)/m)
mass of N2 = 28 * 1.67e-27 kg
not sure if this is the right one - just googled it
triple covalent bond N2 radii = 54e-12 m
I tried to first find the rms speed:
v = x/t
= 50 * 54e-12 / 3 m/s
Then I solved for T (in K):
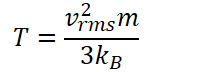
[50 * 54e-12 / 3]^2 * [28 * 1.67e-27] / [3 * 1.38e-23] = 9.14e-22 K
not sure if this is the right one - just googled it
triple covalent bond N2 radii = 54e-12 m
I tried to first find the rms speed:
v = x/t
= 50 * 54e-12 / 3 m/s
Then I solved for T (in K):
[50 * 54e-12 / 3]^2 * [28 * 1.67e-27] / [3 * 1.38e-23] = 9.14e-22 K
Last edited:
