It looks like a lot of your trouble is stemming from the ideas of nucleophilicity and basicity. Let me see if I can clear this up: when either an acid or a base is present in an organic reaction, the first step is *always* going to be a protonation/deprotonation, especially if a strong acid/base is present! Proton transfer is simply the fastest reaction in organic chemistry, so it always happens first--before any nucleophilic additions/substitutions/etc. So where nucleophilicity and basicity are competing, basicity is going to win.
Also, I get the feeling that you're simply memorizing reactions rather than than trying to understand the mechanisms at play. This is the route to doom in O. Chem. Are you using note cards? If you are, you need to throw them away because you're studying incorrectly. When you tackle these problems, your best bet is to start drawing and pushing electrons. Take each problem step by step and only then will they make sense. Now, let's look at some problems! Have a pencil and paper ready to work these out yourself.
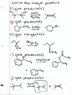
a) SN2 is extremely unlikely to occur on a tertiary substrate, especially in a polar solvent. On the flip-side, you've got a polar protic solvent and a benzylic, tertiary proton. This should be a very strong hint to you that an E1 mechanism occurs. The reason for this is that the intermediate carbocation is going to be quite stable: it will be either on a benzylic, tertiary carbon or on a secondary carbon. E2 may be possible by abstracting a proton from the methyl group with the bromide group leaving, however. However, SN2 on C-2 (a secondary, substituted carbon) is also very unlikely. Your two products will be the elimination products.
b) PBr3 is the right answer. Phosphorus halides, with the exception of PF3 (Fluorine chemistry is always weird and contrary to what you'd expect simply following periodic trends) are good for organic halogenations of alcohols. Notice the double bond in the substrate molecule though. That is the reason why HBr would not give you your desired product in this case (although for alcohols without multiple bonds, this would be an acceptable answer). What happens when HBr encounters an alkene?
c) Your logic is right and you're painfully close to the correct answer, but you missed the details. This isn't a one-step reaction and you have to be careful. Once again, you've got a strong acid (in fact, the definition of a strong acid: sulfuric!) present. What's the first step? (hint: scroll up!) Your sulfuric protonates the O in the epoxide, placing a formal positive charge on it, and basically activates it to ring-opening. The ring opening proceeds by a nucleophilic attack on a carbon by ethanol. Now here's the question for you: which carbon is going to be a better substrate for nucleophilic attack and why? Can you see what you did wrong?
d) Notice the stereochemistry here. E2 is preferred when the leaving group is anti-periplanar to the proton that is being abstracted by the base (here, t-butanoate, which is a very poor nucleophile). It is possible for E2 to occur in the syn-periplanar geometry, as would have to occur in the answer you've shown, but it is much less favorable and has a much higher activation energy than the anti-periplanar geometry. So the product you drew would be a very minor product. Your TA is right: it's E2, but the reaction doesn't happen in a way that produces the product you've drawn.
f) You're making this harder than it should be. Once again, scroll up to the first thing I say in this reply. You've got conc. sulfuric acid in this reaction: it's going to protonate *something,* even if that something is unwilling! However, you've got two willing substrates here: two hydroxyl groups. Let's consider just one of those hydroxyl groups for now, though, and have a pencil and paper to do this. Draw it the protonated hydroxyl group. What kind of carbon is it on? (secondary!) If it decides to bail out on the molecule, would it be a stable leaving group? (It's water, so YES!) This is an E1 mechanism. As a reminder, under acidic conditions, your acid is hydronium (H3O+) and your base is water (H2O). In this mechanism, the acid is just a catalyst, so for every molecule of hydronium you use, you need to regenerate it. How is it regenerated here? Well, the water that just ditched the molecule no comes back to abstract a proton off of C-1! With a bit of electron pushing, a double bond is formed between C-1 and C-2 (the site that the water molecule left). Bingo, hydronium reformed and elimination complete! Now, after this first elimination occurs, a second one could also occur on the other alcohol group. Basically, you get a mixture of products: double bond between C1 and C2; double bond between C3 and C4; double bonds between both C1/C2 and C3/C4. However, notice the symmetry of this molecule: a double bond between C1/C2 is the same as one between C3 and C4. There are two products.
g) E2 and SN2, as a general rule, occur under basic conditions. E1 and SN1, as a general rule, occur under acidic conditions. First step: the oxygen in the ether gets protonated. Now quite a good leaving group (HO+-CH3) has been formed. Guess what it does? It leaves and forms a mostly happy carbocation: it's on a secondary carbon. However, in the overall scheme of things, carbocations are simply intermediates (at least they are in these questions). What's a good nucleophile in this system? Bromide! And voila, you have your product.
i) CH3CO2H is acetic acid. This is occurring under acidic conditions, so the basicity of molecules here isn't important. First step: something's getting protonated. Where are the lone pairs? (On the bromide!) So bromide gets protonated forming H-Br+-, a great leaving group. Now, where's your electron density? On the styrene molecule (the alkene). So, just like in electrophilic addition, this is going to donate electron density to an electron-poor region: i.e., the benzyl carbon with the HBr+ hanging off of it. Push the electrons and the H-Br leaves (and your molecule of acid is regenerated if you think of it as H3O+ doing the initial attack and a molecule of H3O+ and Br- resulting). You've formed a carbon-carbon bond, not a carbon-oxygen-carbon linkage.
j) Now you're just overthinking this. Is water going to react with an alkane? If you dump vaseline into water, what happens? (nothing at all)
Good luck!