doollas
- 4
- 0
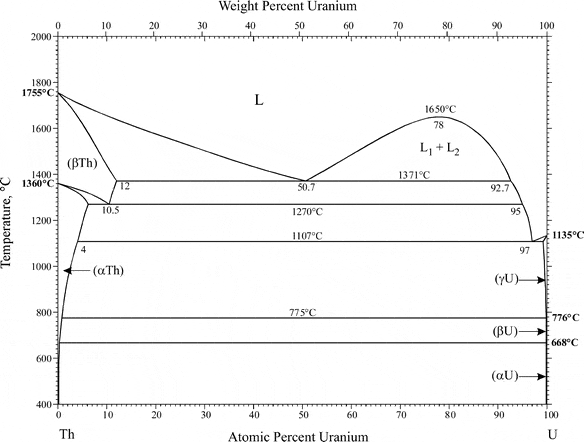
What happens to one of the components when it is at VERY low concentration in a two component alloy? My problem: Uranium decays to thorium. The thorium isotopes it decays to typically have pretty short half lives. During casting, it is well known that thorium fractionates to the slag, crucible, or pretty much anywhere else besides the uranium melt. However, the thorium is at VERY low concentrations (radiogenic Thorium at parts per billion, typically natural thorium isn't there but if it is, we are still talking at maybe a part per million). When talking with a material scientists thus far, they claim that anything at that low of concentration is too dilute to form any separate phases, so what mechanisms could be driving this fractionation? The phase diagram for the two is below. Maybe I'm reading it wrong and this is obvious to some of you, any help would be greatly appreciated!