- #1
stickplot
- 85
- 0
Homework Statement
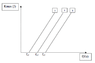
a. Which plot on the graph represents the metal with the lowest work function?
b. What does the slope of the graphs represent? (graph is attached)
Homework Equations
threshold frequency= work function/ planks constant
The Attempt at a Solution
a. the lower the work function, the lower the frequency, so (im still confused about the graph)
so plot 1 is the one with the lowest work function.
b. the slope on the graph shows the amount of energy frequency that it took for a photoelectric effect to occur.
im not to sure about my answers someone help out please.