SchrodingersMu
- 14
- 0
Hi!
I recently did a lab where my group was to create a polymer via free-radical polymerization. We used acrylamide and potassium persulfate. We DID NOT use bis acrylamide crosslinker ( wasnt in our lab manual for some reason.)
I cannot find any web resources that show how acrylamide polymerizes with itself. I know, in this kind of free radical propagation, an initiator ( K persulfate) will change to have a radical under heating, etc. That radical then attacks a double bond. My question is- How would the double bonds in only acrylamide be attacked? We see that there are two options: c=c or c=o. In a typical reaction, the c=c bond is attacked. If the c=o bond is attacked, to me, it looks like it would be more stable, however. From my understanding, oxygen holds charges better than C, and also there would be some resonance from the lone pair on the amine group. If the c=o bond is attacked, though, would there be polymerization propagation?
Our polymer (after being heated) was a clear, semi-flexible thin plastic film
Below is a picture of the typical reaction with crosslinker ( in green, which we didnt have) initiator ( in orange, in the picture it is ammonium instead of potassium persulfate)
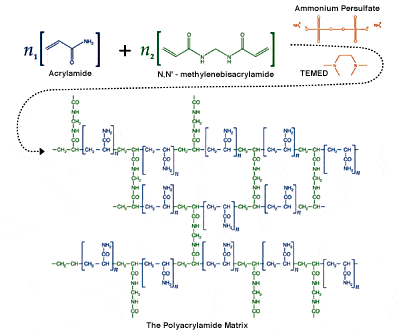
Any input is appreciated!
I recently did a lab where my group was to create a polymer via free-radical polymerization. We used acrylamide and potassium persulfate. We DID NOT use bis acrylamide crosslinker ( wasnt in our lab manual for some reason.)
I cannot find any web resources that show how acrylamide polymerizes with itself. I know, in this kind of free radical propagation, an initiator ( K persulfate) will change to have a radical under heating, etc. That radical then attacks a double bond. My question is- How would the double bonds in only acrylamide be attacked? We see that there are two options: c=c or c=o. In a typical reaction, the c=c bond is attacked. If the c=o bond is attacked, to me, it looks like it would be more stable, however. From my understanding, oxygen holds charges better than C, and also there would be some resonance from the lone pair on the amine group. If the c=o bond is attacked, though, would there be polymerization propagation?
Our polymer (after being heated) was a clear, semi-flexible thin plastic film
Below is a picture of the typical reaction with crosslinker ( in green, which we didnt have) initiator ( in orange, in the picture it is ammonium instead of potassium persulfate)
Any input is appreciated!