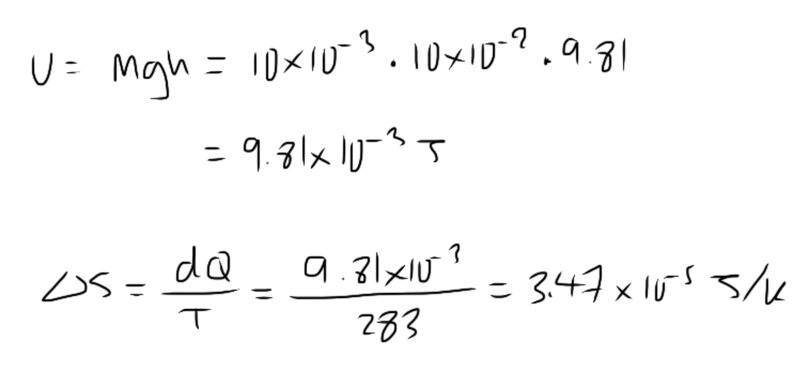Homework Help Overview
The discussion revolves around the probability of a pencil spontaneously jumping 10 cm into the air, considering thermal energy from its surroundings at 15°C. Participants explore the molecular motion of the pencil in different states and the implications of entropy in relation to this spontaneous event.
Discussion Character
- Exploratory, Conceptual clarification, Assumption checking
Approaches and Questions Raised
- Participants question the relationship between molecular motion at rest and in motion, and the implications of thermal energy on spontaneous events. There is also discussion about the connection between gravitational potential energy, entropy, and probability.
Discussion Status
The discussion is ongoing, with participants raising questions about the underlying physics concepts and seeking clarification on how entropy relates to the probability of the event. Some guidance has been offered regarding relevant equations and concepts, but no consensus has been reached.
Contextual Notes
Participants note a lack of clarity on how to connect the concepts of gravitational potential energy and entropy to the probability question posed. There is also mention of constraints related to the course material provided to them.