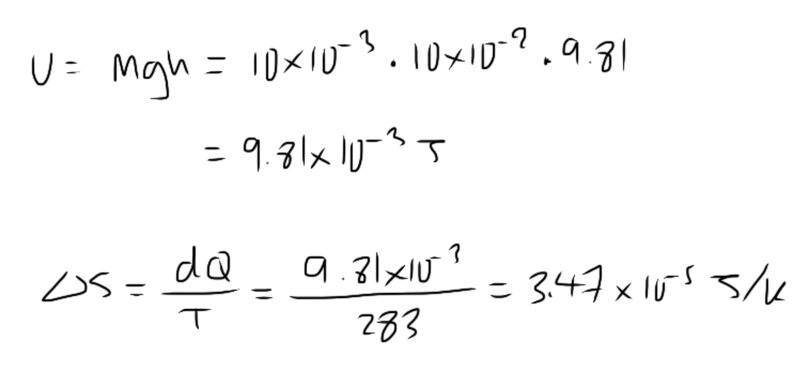SUMMARY
The discussion focuses on the probability of a 10g pencil spontaneously springing 10cm into the air due to thermal energy at 15°C. Participants conclude that while the probability is theoretically 100%, practical constraints, such as the lifetime of the universe, limit this occurrence. Key concepts include the relationship between molecular motion, gravitational potential energy, and entropy, with relevant equations being dS = dQ/T and U = mgh. The conversation emphasizes the need to understand the energy partition function and Boltzmann factor for a complete analysis.
PREREQUISITES
- Understanding of thermodynamics, specifically entropy and energy equations
- Familiarity with the Boltzmann factor and energy partition function
- Basic knowledge of gravitational potential energy calculations
- Concepts of molecular motion and statistical mechanics
NEXT STEPS
- Study the Boltzmann factor and its application in statistical mechanics
- Learn about the energy partition function and its significance in thermodynamics
- Explore the relationship between entropy and probability in physical systems
- Investigate gravitational potential energy calculations in various contexts
USEFUL FOR
This discussion is beneficial for physics students, thermodynamics researchers, and anyone interested in the statistical mechanics of spontaneous events and entropy. It provides insights into the theoretical underpinnings of molecular behavior and energy transformations.