mathzeroh
- 98
- 0
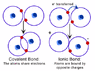
Now obviously, ionic bonds are "weaker" than covalent bonds, but my question is that according to the attached image, you can't necessarily have crystals being formed in a covelant bond as you would in an ionic bond, right? And also, when an ionic bond occurs, what makes it possible for a crystal to form??
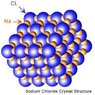
In a NaCl crystal for example, why are the electrons of both Sodium and Chlorine equally attracted to other Na and Cl ions?? I mean, since they are attracted to each other (pretty loosely too I'm assuming), how come when other chlorine ions show up and more Na ions show up, why do they attract to each other and form these crystals? Shouldn't it be only a 1:1 ratio between them?
In a NaCl crystal for example, why are the electrons of both Sodium and Chlorine equally attracted to other Na and Cl ions?? I mean, since they are attracted to each other (pretty loosely too I'm assuming), how come when other chlorine ions show up and more Na ions show up, why do they attract to each other and form these crystals? Shouldn't it be only a 1:1 ratio between them?