maistral
- 235
- 17
- TL;DR
- Deciphering old journal's rate constant notation.
So I was looking for some rate constants for a certain reaction and I found these:
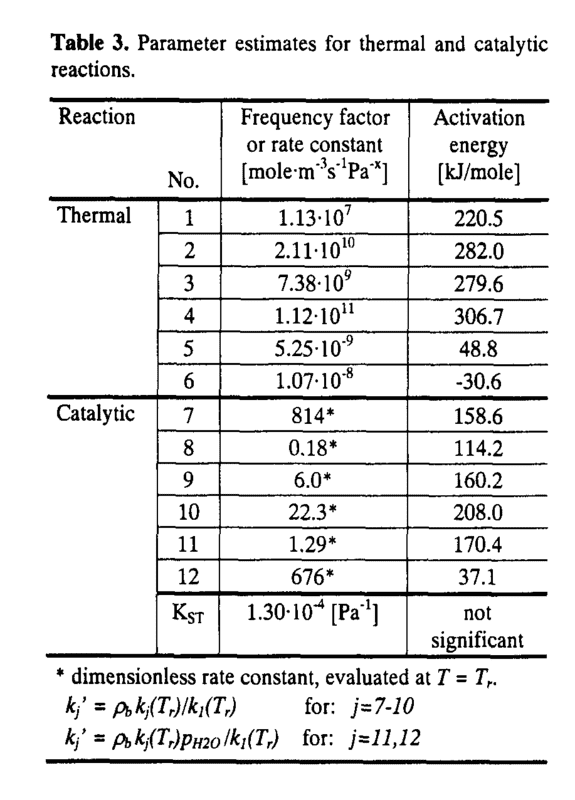
So I wanted to try them. The problem is, I have no idea how to correctly write the rate constants. Reactions number 1 to 6 is a no-brainer as it's simple Arrhenius. My problem is for rate constants 7 to 12. I am aware of evaluating T at Tr, the problem is I have no idea how to write the entire Arrhenius form and I am somehow unable to wrap my head around it. Can someone give me an idea how? Thanks.
EDIT: I'm guessing, but are these correct? I follow the equation then I compute the preexponential? For rate constant 7, this:
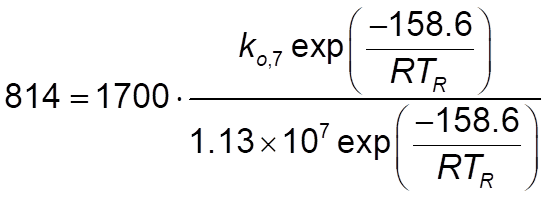
and for rate constant 12, this:
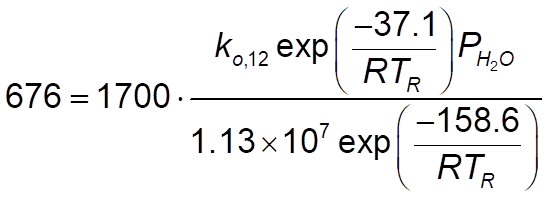
So I wanted to try them. The problem is, I have no idea how to correctly write the rate constants. Reactions number 1 to 6 is a no-brainer as it's simple Arrhenius. My problem is for rate constants 7 to 12. I am aware of evaluating T at Tr, the problem is I have no idea how to write the entire Arrhenius form and I am somehow unable to wrap my head around it. Can someone give me an idea how? Thanks.
EDIT: I'm guessing, but are these correct? I follow the equation then I compute the preexponential? For rate constant 7, this:
and for rate constant 12, this:
Last edited: