crysien
- 1
- 0
I am a little confused by something by something in my physical chemistry textbook.
If two measurable quantities do not commute, then an uncertainty relation exists for them. Kinetic energy and position do not commute, and the expectation value for linear momentum in a 1-D particle in a box is zero. However, the uncertainty relation for KE and position says that:
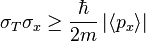
Obviously, the uncertainty can't be zero, but I don't see why this equation is correct and I haven't found anything working out the actual integral to reach this result online. Could someone please explain?
If two measurable quantities do not commute, then an uncertainty relation exists for them. Kinetic energy and position do not commute, and the expectation value for linear momentum in a 1-D particle in a box is zero. However, the uncertainty relation for KE and position says that:
Obviously, the uncertainty can't be zero, but I don't see why this equation is correct and I haven't found anything working out the actual integral to reach this result online. Could someone please explain?