- #1
patric44
- 296
- 39
- Homework Statement
- i'am trying to find the volume of the nucleus to the volume of the electron (for hydrogen )
- Relevant Equations
- R = R. A^1/3 the radius of the nucleus
re = 2.8 * 10^-15 m
i'am trying to find the ratio between the volume of the nucleus for the hydrogen atom to its electron , but when i try to use the previous equations it seems wrong as i'am getting a low number like if the electron is bigger .
i used the the classical electron radius as it was the only thing that i could find :
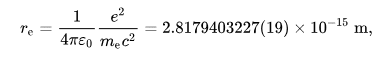
and the radius of the nucleus is obtained from the equation:
R = Ro A1/3 and as Ro = 1.2 * 10-15 m
now the radius of the electron is bigger ?!
is this equation not valid for small atoms like hydrogen ?
is there any other approach for the problem ?
i used the the classical electron radius as it was the only thing that i could find :
and the radius of the nucleus is obtained from the equation:
R = Ro A1/3 and as Ro = 1.2 * 10-15 m
now the radius of the electron is bigger ?!
is this equation not valid for small atoms like hydrogen ?
is there any other approach for the problem ?