- #1
Trettman
- 4
- 0
"Calculated the total magnetic moment of a hydrogen atom in the ground state in a weak magnetic field which arises from hyper fine splitting of the ground state. How many beams does hydrogen produce from a Stern-Gerlach analyzer with a weak magnetic field?"
I've calculated the magnetic moments from the hyper fine structure splitting to be
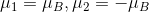
but I don't really know how to get the total magnetic moment of hydrogen. My guess so far is to compute the vector sum of
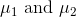 "point". How do you "vectorize" them?
"point". How do you "vectorize" them?
I'm also kind of confused as to how the magnetic moment of a particle is calculated. For example, the spin magnetic moment of a particle is
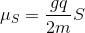 .
.
Since S is an operator, this also means that the magnetic moment,
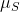 , is an operator? This kind of confuses me. Is the "length" of
, is an operator? This kind of confuses me. Is the "length" of
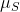 given by the root of the eigenvalues of
given by the root of the eigenvalues of
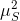 , which would be
, which would be
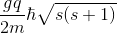 ?
?
Lastly, I'm guessing that to answer the question "How many beams does hydrogen produce from a Stern-Gerlach analyzer with a weak magnetic field?", you just count the number of different values you get for the total magnetic moment?
Thanks!
I've calculated the magnetic moments from the hyper fine structure splitting to be
but I don't really know how to get the total magnetic moment of hydrogen. My guess so far is to compute the vector sum of
- the electron's spin magnetic moment
- the electron's orbital magnetic moment
- the proton's spin magnetic moment
- the magnetic moment that arises from the hyper fine structure (),
I'm also kind of confused as to how the magnetic moment of a particle is calculated. For example, the spin magnetic moment of a particle is
Since S is an operator, this also means that the magnetic moment,
Lastly, I'm guessing that to answer the question "How many beams does hydrogen produce from a Stern-Gerlach analyzer with a weak magnetic field?", you just count the number of different values you get for the total magnetic moment?
Thanks!