khkwang
- 57
- 0
For simplicity sake, I'm assuming an electron is traveling in just one direction (x).
From what I understand the square of the electron's wave function is proportional to the probability of finding it at x.
But there's a point in every period that the amplitude is zero, which means the probability of finding it there is zero as well.
So doesn't that mean that the probability of an electron existing over a range of x grows and shrinks back to zero periodically?
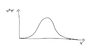
example for probability p(x):
p(<0)=0, p(0)=0, p(1)=1, p(2)=0, p(3)=4, p(4)=0, p(5)=9, p(6)=0, p(>6)=0
Am I interpreting this properly?
From what I understand the square of the electron's wave function is proportional to the probability of finding it at x.
But there's a point in every period that the amplitude is zero, which means the probability of finding it there is zero as well.
So doesn't that mean that the probability of an electron existing over a range of x grows and shrinks back to zero periodically?
example for probability p(x):
p(<0)=0, p(0)=0, p(1)=1, p(2)=0, p(3)=4, p(4)=0, p(5)=9, p(6)=0, p(>6)=0
Am I interpreting this properly?