- #1
Guilherme Franco
- 10
- 1
- TL;DR Summary
- I'm trying to deconvolute IR absorption spectra and find (or at least get some idea) of the relative proportion of (known) bonds in samples
Hi
I'm preparing some BCNO phosphor samples. Their basic structure is hexagonal Boron Nitride (h-BN) but it's doped with carbon and oxygen. The simplest BCNO phosphors are usually made from urea and boric acid alone, this already produces a BN structure with C and O impurities.
I'm trying to use IR absorption spectra from several samples to find the optimal B/N (boric acid and urea) mass fraction in a certain preparation condition. And when I say optimal I mean the ones that maximize the amount of B-N bonds relative to the others found in the samples.
So I got the IR absorption data and now I'm trying to deconvolute the data using several gaussian peaks (manually, as no fitting algorithm would ever get to optimize more than 80 variables at once from scratch).
I'm still working in the first one and I already had to use 25 peaks to get started. This gif below shows the data (in red) and the fit so far (in blue):
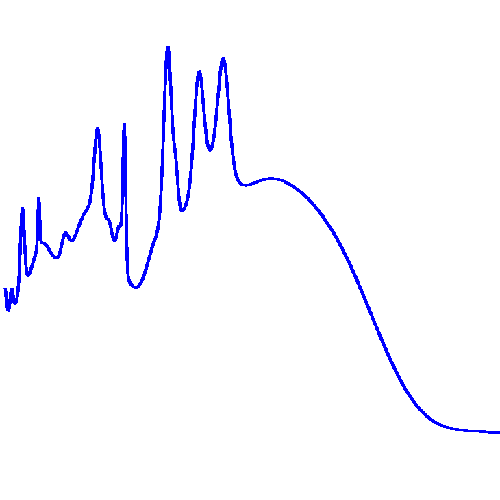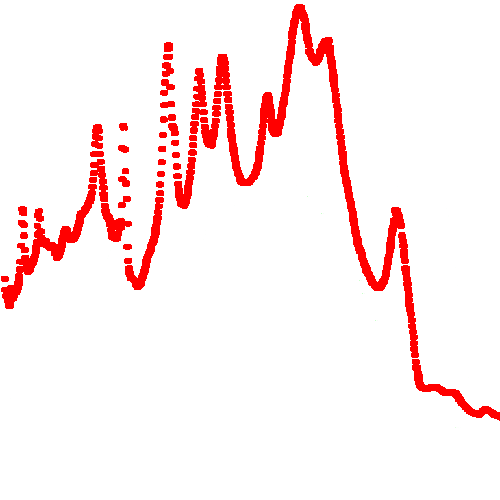
And as you can see on it, I had to use wider gaussians to form a "base" and then narrower ones for the most prominent peaks.
However, my question is about the interpretation I'll be able to make from that. Identifying the narrow and strong peaks is quite feasible as there is data in BCNO articles about their identification, but I'm questioning myself about the possible relationships between the bonds I'm interested in quantifying and those broader peaks in the "base" I made.
Technically those broad peaks are also the result of many convoluted peaks that I'm "leaving convoluted" as I don't need to deconvolute them to model any of the prominent and narrow peaks that appear in my samples (being that only those narrow peaks are associated to the bonds I'm interested). But they do affect the area, width and center of the gaussians I have to use for the narrow peaks a bit (not much in many cases, as nearly any other "base" would be similar to get the fit working).
So, my question is: Can I just consider the area of the narrow gaussians to quantify the (relative) amount of bonds (associated to that peak) and disregard the "base" that I used? Is this a valid method?
Thanks!
I'm preparing some BCNO phosphor samples. Their basic structure is hexagonal Boron Nitride (h-BN) but it's doped with carbon and oxygen. The simplest BCNO phosphors are usually made from urea and boric acid alone, this already produces a BN structure with C and O impurities.
I'm trying to use IR absorption spectra from several samples to find the optimal B/N (boric acid and urea) mass fraction in a certain preparation condition. And when I say optimal I mean the ones that maximize the amount of B-N bonds relative to the others found in the samples.
So I got the IR absorption data and now I'm trying to deconvolute the data using several gaussian peaks (manually, as no fitting algorithm would ever get to optimize more than 80 variables at once from scratch).
I'm still working in the first one and I already had to use 25 peaks to get started. This gif below shows the data (in red) and the fit so far (in blue):
And as you can see on it, I had to use wider gaussians to form a "base" and then narrower ones for the most prominent peaks.
However, my question is about the interpretation I'll be able to make from that. Identifying the narrow and strong peaks is quite feasible as there is data in BCNO articles about their identification, but I'm questioning myself about the possible relationships between the bonds I'm interested in quantifying and those broader peaks in the "base" I made.
Technically those broad peaks are also the result of many convoluted peaks that I'm "leaving convoluted" as I don't need to deconvolute them to model any of the prominent and narrow peaks that appear in my samples (being that only those narrow peaks are associated to the bonds I'm interested). But they do affect the area, width and center of the gaussians I have to use for the narrow peaks a bit (not much in many cases, as nearly any other "base" would be similar to get the fit working).
So, my question is: Can I just consider the area of the narrow gaussians to quantify the (relative) amount of bonds (associated to that peak) and disregard the "base" that I used? Is this a valid method?
Thanks!