MexChemE
- 237
- 54
Hi, PF! I recently solved a problem from BSL which asked to analyze the following system used for determining the diffusivity of a binary mixture of gases.
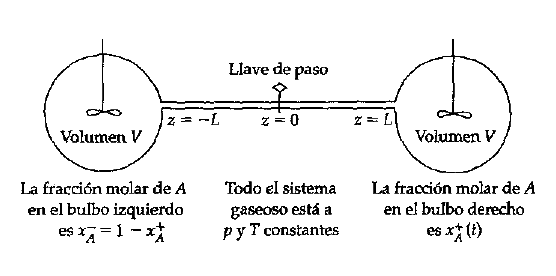
The left portion of the system, from the left bulb up to the stopcock at the middle of the tube, is filled with gas A. The right portion of the system is filled with gas B. At t = 0, the stopcock is opened a the gases start to diffuse. This is a quasi-steady state process. First we derive an expression for the molar flux of A through the tube using a steady state molar balance, and then we make an unsteady state molar balance for species A on the left bulb. The goal is to obtain an expression for x_A^+ as a function of time. The function is
\ln \left(\frac{\tfrac{1}{2} - x_A^+}{\tfrac{1}{2}} \right) = - \frac{SD_{AB} t}{LV}
Where S is the cross-section area of the tube. What got my attention is that the last part of the problem asked to suggest a method of plotting the experimental data in order to find the diffusivity. What I suggested was to define
y = \ln \left(\frac{\tfrac{1}{2} - x_A^+}{\tfrac{1}{2}} \right)
m = - \frac{SD_{AB}}{LV}
Then we can make a linear regression with the data from the experiment and find the slope, then obtain D_{AB} from it.
My actual doubt lies within the implementation of the experiment. Specifically, calculating the mole fraction of gas A in the mixture. Is there a way to do that without using advanced equipment? I.e. can you calculate the mole fraction of A in the mixture using only basic lab equipment?
Thanks in advance for any input!
The left portion of the system, from the left bulb up to the stopcock at the middle of the tube, is filled with gas A. The right portion of the system is filled with gas B. At t = 0, the stopcock is opened a the gases start to diffuse. This is a quasi-steady state process. First we derive an expression for the molar flux of A through the tube using a steady state molar balance, and then we make an unsteady state molar balance for species A on the left bulb. The goal is to obtain an expression for x_A^+ as a function of time. The function is
\ln \left(\frac{\tfrac{1}{2} - x_A^+}{\tfrac{1}{2}} \right) = - \frac{SD_{AB} t}{LV}
Where S is the cross-section area of the tube. What got my attention is that the last part of the problem asked to suggest a method of plotting the experimental data in order to find the diffusivity. What I suggested was to define
y = \ln \left(\frac{\tfrac{1}{2} - x_A^+}{\tfrac{1}{2}} \right)
m = - \frac{SD_{AB}}{LV}
Then we can make a linear regression with the data from the experiment and find the slope, then obtain D_{AB} from it.
My actual doubt lies within the implementation of the experiment. Specifically, calculating the mole fraction of gas A in the mixture. Is there a way to do that without using advanced equipment? I.e. can you calculate the mole fraction of A in the mixture using only basic lab equipment?
Thanks in advance for any input!