Sabra_a
- 33
- 6
- Homework Statement
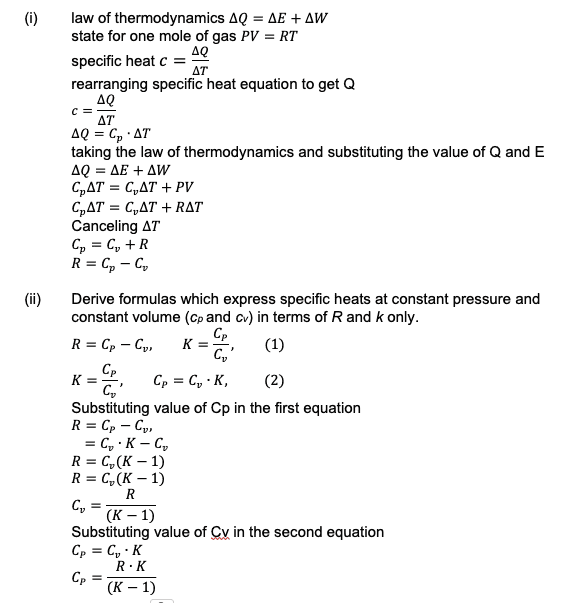
- 1) Using the first law of thermodynamics ∆Q=∆E+∆W (Q – heat added to the system, E – internal energy of the system, W – work done by the system), the equation of state for one mole of gas PV=RT (P - pressure, V – volume, R - universal gas constant, T - temperature), and the definition of the specific heat c=∆Q/∆T derive the following equation for universal gas constant: R= cp- cv (cp and cv are specific heats at constant pressure and constant volume correspondingly).
2) The specific heats ratio or adiabatic exponent is equal by definition to k= cp/cv. Derive formulas which express specific heats at constant pressure and constant volume (cp and cv) in terms of R and k only.
- Relevant Equations
- First law of thermodynamics
Ideal gas equation
Specific heat
In the first question should I remove the delta and put d or that doesn't make a difference and on the second question should I substitute the values of R, K, Cv and Cp or that's not required I'm not really sure how correct is my answer to the second question