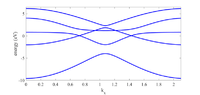
Valence and conduction bands in five band Hamiltonian
- Context: Undergrad
- Thread starter Mohammad-gl
- Start date
-
- Tags
- Band Conduction Hamiltonian
Click For Summary
Discussion Overview
The discussion revolves around the identification of valence and conduction bands in a five band Hamiltonian model, specifically in the context of a band structure plot for b12-borophene. Participants seek clarification on the nature of these bands and any special nomenclature associated with them, as well as the material's classification as an insulator, semiconductor, or metal.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
Main Points Raised
- Some participants propose that energy levels above 0 eV indicate conduction bands, while those below indicate valence bands.
- There is confusion regarding the band structure diagram, which appears to show the material transitioning between insulator, semiconductor, and metal states as one moves across the plot.
- One participant identifies the material in question as b12-borophene.
- Another participant suggests that the band diagram indicates the material is a metal, with an indirect bandgap for certain directions and a direct bandgap for others.
- References to external articles and studies are provided to support claims about the material's properties and band structure.
Areas of Agreement / Disagreement
Participants express differing views on the classification of the bands and the material's behavior, indicating that multiple competing perspectives remain without a consensus.
Contextual Notes
The discussion includes references to specific articles that may contain additional insights, but the interpretations of the band structure and material properties remain unresolved and dependent on the context of the diagrams presented.
Similar threads
- · Replies 11 ·
- · Replies 4 ·
- · Replies 24 ·
- · Replies 2 ·
- · Replies 6 ·
- · Replies 4 ·
- · Replies 7 ·
- · Replies 6 ·
- · Replies 4 ·
- · Replies 0 ·