tonyjk
- 227
- 3
Hello,
Consider this exemple:
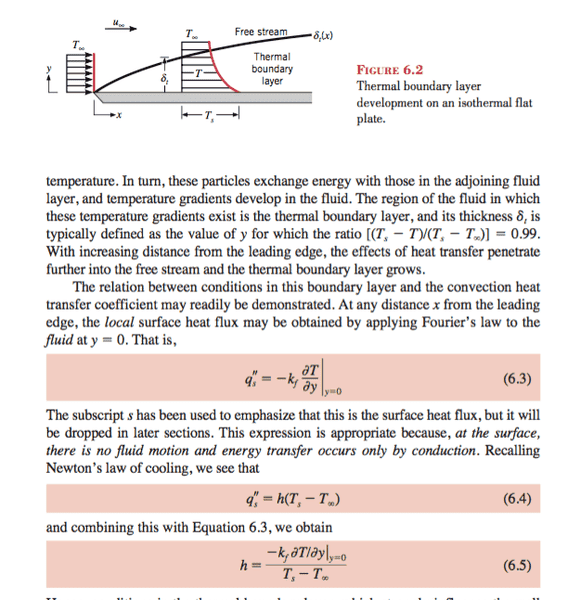
At y = 0 It is said that the heat is transferred by conduction because the fluid has a velocity equal to 0 (no-slip conditions). But the temperature of the fluid for y=0 is equal to Ts(temperature of the surface) so how come there's a gradient in the temperature? Do they mean there's gradient at y=0 just above the layer of the fluid having a temperature equal to Ts? Another question, the conduction heat transfer at y = 0 is happening for 1s (J/S = W) but during this second the fluid is passing and it is not static like in a wall that issue is confusing me. Even the exchange of energy between the fluid that is not on the surface, this fluid is flowing and heat is transferred each second by qs = h(Ts-Tinf) and on each position x we have a gradient of temperature that is independent of the time. I am very confused about the steady state heat transfer by convection.
Hope someone can clarify it
Thank you
Consider this exemple:
At y = 0 It is said that the heat is transferred by conduction because the fluid has a velocity equal to 0 (no-slip conditions). But the temperature of the fluid for y=0 is equal to Ts(temperature of the surface) so how come there's a gradient in the temperature? Do they mean there's gradient at y=0 just above the layer of the fluid having a temperature equal to Ts? Another question, the conduction heat transfer at y = 0 is happening for 1s (J/S = W) but during this second the fluid is passing and it is not static like in a wall that issue is confusing me. Even the exchange of energy between the fluid that is not on the surface, this fluid is flowing and heat is transferred each second by qs = h(Ts-Tinf) and on each position x we have a gradient of temperature that is independent of the time. I am very confused about the steady state heat transfer by convection.
Hope someone can clarify it
Thank you