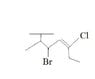
What Is the Correct Chemical Structure for Hexane?
- Thread starter ahazen
- Start date
-
- Tags
- Structure
Click For Summary
Discussion Overview
The discussion revolves around identifying and naming the chemical structure of hexane, with participants exploring various naming conventions and structural interpretations. The scope includes chemical nomenclature and structural analysis.
Discussion Character
- Exploratory, Technical explanation, Debate/contested
Main Points Raised
- One participant seeks assistance in naming a structure they believe is hexane, proposing names like 2-methyl butane and 3-bromo-4,5-ene.
- Another participant asserts that the structure is not hexane, questioning if a "T" in the structure indicates isopropyl.
- A different participant expresses uncertainty about the meaning of "T" and suggests that if it is not hexane, it must be octane.
- Another participant proposes that the structure resembles an alkene attached to a solid support or as a side chain for a polymer, indicating that naming should end with "-yl."
Areas of Agreement / Disagreement
Participants do not reach a consensus on the correct identification or naming of the structure, with multiple competing views and interpretations presented.
Contextual Notes
There are uncertainties regarding the meaning of the "T" in the structure, and participants reference their inability to find the structure in their books, indicating potential limitations in their resources.
Who May Find This Useful
Chemistry students, educators, and professionals interested in organic chemistry and chemical nomenclature may find this discussion relevant.
Similar threads
- · Replies 2 ·
- · Replies 1 ·
- · Replies 1 ·
- · Replies 1 ·
- · Replies 1 ·