Samar A
- 10
- 0
Hi,
The x-ray consists of the bremsstrahlung spectrum and the characteristic spectrum. We can get x-rays by using Coolidge tube where there are an applied voltage on the tube between the filament and the target. The bremsstrahlung is depeding on the PD between the filament and the target, but it is produced anyways. The characteristic spectrum requires a high PD to be produced.
I wonder how could we determine the less voltage is sufficient for the emergence of characteristic spectrum by using a diagram of the two spectra, like this:
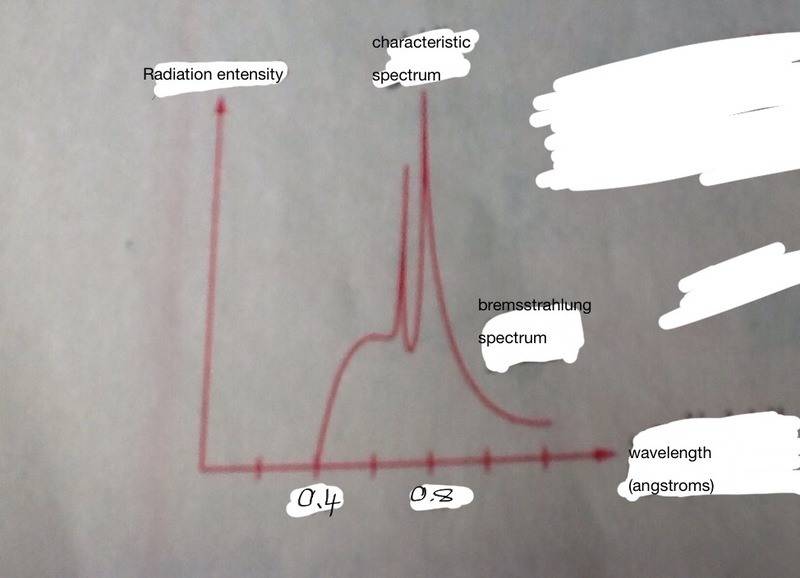
(Note: the information on the photo were not clear so I deleted them and wrote them again using my cellphone)
Anyways, in my textbook it tried to give the less voltage needed for the characteristic spectrum, and it did this:
E=hc/λ =(6.625*10^-34)*(3*10^8)/ (0.8*10^-10)
=2.48*10^-15 J
And then, because E=eV:
2.48*10^-15= (1.6*10^-19) *V
So, V= 1.55*10^4 Volts.
I am not convinced with the way it used and I don't know how to determine it myself, either.
Could someone explain me way it used the value (0.8*10^-10) in the first part of the solution, because I don't think it is the longest wavelength the characteristic would appear.
The x-ray consists of the bremsstrahlung spectrum and the characteristic spectrum. We can get x-rays by using Coolidge tube where there are an applied voltage on the tube between the filament and the target. The bremsstrahlung is depeding on the PD between the filament and the target, but it is produced anyways. The characteristic spectrum requires a high PD to be produced.
I wonder how could we determine the less voltage is sufficient for the emergence of characteristic spectrum by using a diagram of the two spectra, like this:
(Note: the information on the photo were not clear so I deleted them and wrote them again using my cellphone)
Anyways, in my textbook it tried to give the less voltage needed for the characteristic spectrum, and it did this:
E=hc/λ =(6.625*10^-34)*(3*10^8)/ (0.8*10^-10)
=2.48*10^-15 J
And then, because E=eV:
2.48*10^-15= (1.6*10^-19) *V
So, V= 1.55*10^4 Volts.
I am not convinced with the way it used and I don't know how to determine it myself, either.
Could someone explain me way it used the value (0.8*10^-10) in the first part of the solution, because I don't think it is the longest wavelength the characteristic would appear.