David89
- 19
- 4
Homework Statement
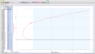
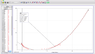
I've been doing an experiment where I measure the water volume in a glass bottle as I manipulate the temperature from room temp (24.8 degrees celsius) to 0 degrees celsius, and then back up to room temp.
I know that water behaves uniquely from 0 to 4 degrees celsius, it contracts instead of expands, but, as the water was increasing in temperature, from 0 degrees to 15 degrees, I placed the bottle in a tub of hot water measuring approx 35 degrees.
Now the water inside the bottle contracted a significant amount before beginning to expand again.
Homework Equations
I have uploaded a pic of my graph, and you can see that at 15 degrees the graph does some weird stuff and then continues on its way
The Attempt at a Solution
I really don't know why the rapid temperature change caused the water to contract initially.