mjmnr3
- 5
- 0
- Homework Statement
- 1.8 Consider the strong interaction ##\pi^{-} d \rightarrow n n,## where ##d## is a spin- 1 S-wave bound state of a proton and a neutron called the deuteron and the initial pion is at rest. Deduce the intrinsic parity of the negative pion.
- Relevant Equations
- $$\hat{P}\psi_{nlm}=p(-1)^l \psi_{nlm}$$
I looked in the instructor solutions, which are given by:
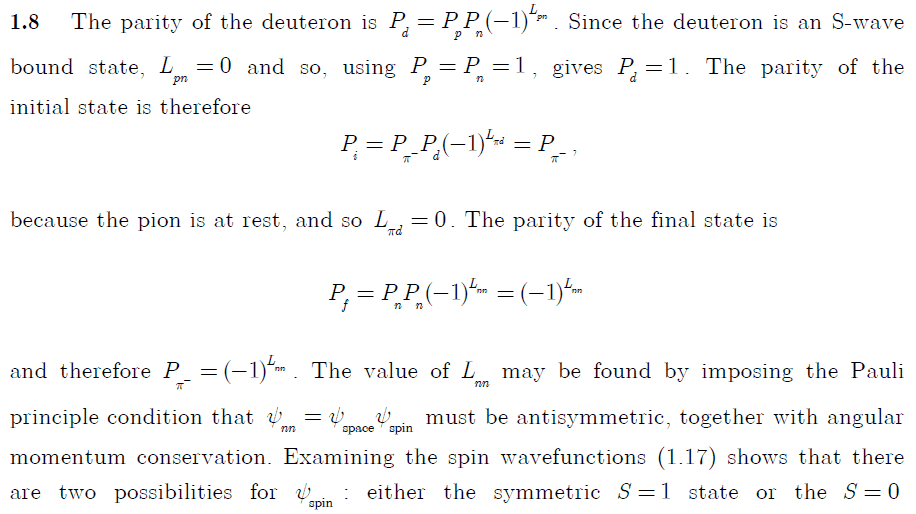
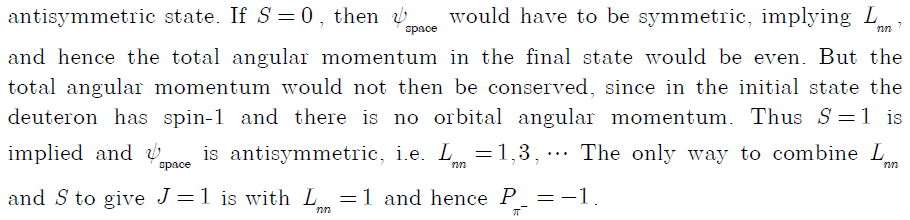
But I don't quite understand the solution, so I hope you can help me understand it.
First. Why do we even know we are working with wavefunctions with the quantum numbers n,l,m? Don't we only get these quantum numbers if the particles we're working with, is in a spherical symmetric potential?
So in other words, why do we need to consider the quantum number l (angular momentum)? Is that because strong force gives rise to a spherical potential?
When using the parity operator, why do we get the the combined angular momentum ##(-1)^{L_{\pi d}}## and not just: ##(-1)^{L_{\pi}}\cdot (-1)^{L_d}##. Can I always just couple the angular momentum when working with multiple particle wavefunctions, and assume operators only works on the coupled angular momentum?
The most important question, is how the angular momentum relates to the symmetry of a wave function.
Why does a symmetric space wavefunction ##\psi_{space}=symmetric## implies that the angular momentum ##L_{nn}## has to be even?
And the opposite. Why does an antisymmetric wavefunction imply uneven angular momentum?
But I don't quite understand the solution, so I hope you can help me understand it.
First. Why do we even know we are working with wavefunctions with the quantum numbers n,l,m? Don't we only get these quantum numbers if the particles we're working with, is in a spherical symmetric potential?
So in other words, why do we need to consider the quantum number l (angular momentum)? Is that because strong force gives rise to a spherical potential?
When using the parity operator, why do we get the the combined angular momentum ##(-1)^{L_{\pi d}}## and not just: ##(-1)^{L_{\pi}}\cdot (-1)^{L_d}##. Can I always just couple the angular momentum when working with multiple particle wavefunctions, and assume operators only works on the coupled angular momentum?
The most important question, is how the angular momentum relates to the symmetry of a wave function.
Why does a symmetric space wavefunction ##\psi_{space}=symmetric## implies that the angular momentum ##L_{nn}## has to be even?
And the opposite. Why does an antisymmetric wavefunction imply uneven angular momentum?