- #1
vcsharp2003
- 897
- 176
- Homework Statement
- I am trying to explain a common phenomenon in nature. The phenomenon is that warm air in room rises in a room and colder air at the top of the room settles down closer to the floor of the room.
- Relevant Equations
- ##\rho = \frac {m} {v}##, where ##\rho## is density of material and ##m## , ##v## are mass and volume of a certain amount of the material
My answer given below seems incomplete.
Since warm air causes the air to expand in volume, so its density becomes less as compared to the colder air at the top of the room. After this, I generally find all books saying the less dense air rises and more dense air from top comes down and therefore this phenomenon occurs in nature.
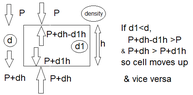
But, how would I explain why less dense air rises and more dense air falls. I have tried to explain it using the diagram below which shows two cubes of air at the top and bottom of the room. The arrows for each cube indicate the pressure acting on the cube from all directions. Wouldn't the force due to pressure on each face of the cube be balanced by the force due to pressure on the opposite face of the cube? If yes, then I cannot explain why the cube of air at the bottom would rise since it needs to have a net upward force acting on it in order for it to rise. If the upward pressure is greater than the downward pressure on the bottom cube, then I could say that the less dense cube of air will rise since it would end up having a net upward force.
Or perhaps, there is another explanation for this.

Since warm air causes the air to expand in volume, so its density becomes less as compared to the colder air at the top of the room. After this, I generally find all books saying the less dense air rises and more dense air from top comes down and therefore this phenomenon occurs in nature.
But, how would I explain why less dense air rises and more dense air falls. I have tried to explain it using the diagram below which shows two cubes of air at the top and bottom of the room. The arrows for each cube indicate the pressure acting on the cube from all directions. Wouldn't the force due to pressure on each face of the cube be balanced by the force due to pressure on the opposite face of the cube? If yes, then I cannot explain why the cube of air at the bottom would rise since it needs to have a net upward force acting on it in order for it to rise. If the upward pressure is greater than the downward pressure on the bottom cube, then I could say that the less dense cube of air will rise since it would end up having a net upward force.
Or perhaps, there is another explanation for this.
Last edited: