Discussion Overview
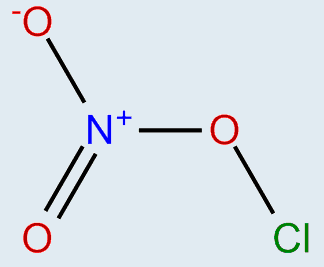
The discussion revolves around the molecular structure of chlorine nitrate (ClONO2) and the implications of its charge distribution, particularly concerning the nitrogen and oxygen atoms. Participants explore theoretical models, bonding characteristics, and the representation of charges in chemical formulas.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Mathematical reasoning
Main Points Raised
- Some participants question the presence of a positive charge on nitrogen in chlorine nitrate and why it is not reflected in the formula ClONO2.
- Others note that a +1 charge and a -1 charge can coexist in the same molecule, resulting in an overall neutral charge.
- A participant expresses skepticism about the current theoretical understanding, suggesting that chlorine might form multiple bonds with oxygen and nitrogen differently than represented.
- There is a discussion about the maximum oxidation state of chlorine, with some proposing +7 and others suggesting that the oxidation state could exceed this maximum based on bonding assumptions.
- Formal charge calculations are suggested as a more appropriate method for evaluating the bonding situation in chlorine nitrate.
- Participants analyze bond lengths from external sources, noting that discrepancies in bond lengths may indicate the nature of bonding and charge distribution.
- One participant introduces the concept of coordinate covalent bonds to explain how nitrogen shares electrons with oxygen, leading to a positive charge on nitrogen.
- Another participant challenges the interpretation of bond lengths, suggesting that the presence of resonance or delocalization might explain the observed bond characteristics.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the interpretation of the molecular structure and charge distribution in chlorine nitrate. Multiple competing views and hypotheses are presented, reflecting uncertainty and differing interpretations of the bonding and charge characteristics.
Contextual Notes
Participants acknowledge limitations in their theoretical understanding and the complexity of Lewis structures, which may not fully capture the nuances of molecular bonding. There are also unresolved questions regarding the implications of bond lengths and the nature of charge distribution.