Terrysv
- 23
- 1
Hello everyone,
I am going to try show the advantage of using liquid vapor phase equilibrium in my design compared to the expansion of a more common diesel engine using Amonton's law. All gases expand at about the same rate when heated (33%) and water expands at about 1600 times when boiled requiring a lot less energy is to produce an equal pressure change in a quantity of saturated water.
If a piston in a typical diesel engine compresses a volume of air to 41 bar at 450°C and then the combustion event brings the pressure up to 70 bar at 1667°C. Therefore using Amonton's law we know that enough fuel needs to be spent to bring the temperature of the volume of compressed gas up 1217°C to get the pressure desired. A liquid to vapor phase change will need an increase of 33.5°C to convert enough saturated water into the same volume and pressure of saturated vapor.
The benefit of using a phase change in a closed cycle engine is that most of the thermal energy is retained in the condensation and this retained energy is able to get out of its own way during the compression part (4) of the cycle.
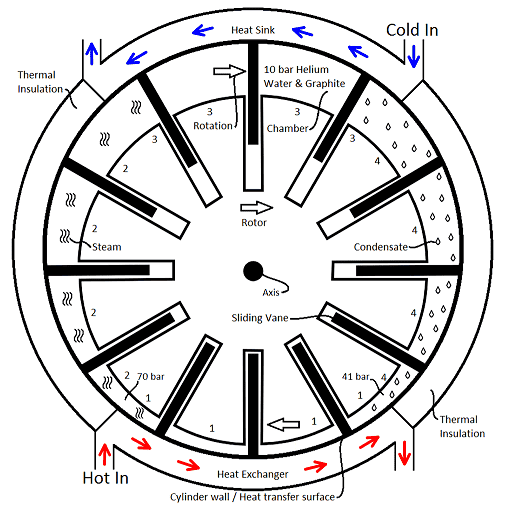
Description of the cycle and the activity in a sample chamber:
Section 1 (4:30 to 7:30) the chamber path, has a small volume with a low volume change as the chamber moves past in a clockwise direction.
A chamber of compressed air, with a minimal quantity of saturated water present is preheated to 41 bar at 253.3°C. The chamber will accumulate an additional 33.5°C as it passes by the adjacent reverse flow heat exchange, bringing the contents up to 70 bar at 286.8°C, replacing only the quantity of thermal energy that was rejected on the previous cycle. The additional pressure gained is produced by the quantity of saturated water becoming a compressible water vapor.
Section 2 (7:30 to 10:30) of the chamber path, has an increasing volume.
The 70 bar at 287°C of pressure is declining from adiabatic expansion.
Section 3 (10:30 to 1:30) of the chamber path, has a large volume with a low volume change as each chamber moves past in a clockwise direction.
The chamber pressure is dropping as the water vapor is returning to a liquid state as the thermal energy is rejected to the reverse flow heat sink. Without having an exhaust valve all the remaining thermal energy is retained in the condensate.
Section 4 (1:30 to 4:30) of the chamber path, has a declining volume.
The chamber having lost a quantity of thermal energy is now gaining pressure and thermal energy from adiabatic compression arriving at the cycle starting point with 41 bar at 253°C.
I am going to try show the advantage of using liquid vapor phase equilibrium in my design compared to the expansion of a more common diesel engine using Amonton's law. All gases expand at about the same rate when heated (33%) and water expands at about 1600 times when boiled requiring a lot less energy is to produce an equal pressure change in a quantity of saturated water.
If a piston in a typical diesel engine compresses a volume of air to 41 bar at 450°C and then the combustion event brings the pressure up to 70 bar at 1667°C. Therefore using Amonton's law we know that enough fuel needs to be spent to bring the temperature of the volume of compressed gas up 1217°C to get the pressure desired. A liquid to vapor phase change will need an increase of 33.5°C to convert enough saturated water into the same volume and pressure of saturated vapor.
The benefit of using a phase change in a closed cycle engine is that most of the thermal energy is retained in the condensation and this retained energy is able to get out of its own way during the compression part (4) of the cycle.
Description of the cycle and the activity in a sample chamber:
Section 1 (4:30 to 7:30) the chamber path, has a small volume with a low volume change as the chamber moves past in a clockwise direction.
A chamber of compressed air, with a minimal quantity of saturated water present is preheated to 41 bar at 253.3°C. The chamber will accumulate an additional 33.5°C as it passes by the adjacent reverse flow heat exchange, bringing the contents up to 70 bar at 286.8°C, replacing only the quantity of thermal energy that was rejected on the previous cycle. The additional pressure gained is produced by the quantity of saturated water becoming a compressible water vapor.
Section 2 (7:30 to 10:30) of the chamber path, has an increasing volume.
The 70 bar at 287°C of pressure is declining from adiabatic expansion.
Section 3 (10:30 to 1:30) of the chamber path, has a large volume with a low volume change as each chamber moves past in a clockwise direction.
The chamber pressure is dropping as the water vapor is returning to a liquid state as the thermal energy is rejected to the reverse flow heat sink. Without having an exhaust valve all the remaining thermal energy is retained in the condensate.
Section 4 (1:30 to 4:30) of the chamber path, has a declining volume.
The chamber having lost a quantity of thermal energy is now gaining pressure and thermal energy from adiabatic compression arriving at the cycle starting point with 41 bar at 253°C.