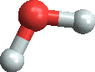
Well, water is composed of three atoms two hydrogen and one oxygen. So a molecule looks likes this:
-When the temperature decreases the molecules start slowing down.
-This causes the volume to decrease and density to increase until 4°C while its still in liquid state.
-After this the molecules start crystallizing in a cage like structure by hydrogen bonding. Hydrogen bonding is a weak molecular interaction between the oxygen of one molecule and Hydrogen of another.
-In the crystallization process the density decreases and volume increases as the H-bonds push molecules apart to maintain a stable crystal lattice.
-The differences can be seen in this image [left is liquid and right is ice]:
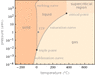
The temperature and pressure relationship of water is given by: