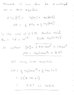Studiot said:
As promised here is a derivation of the H-H equation, establishing why the OH- concentration is crucial and using it to calculate the initial pH of ).1M acetic acid as requested.
The calculation is compared with another method of achieving the pH.
This method will only work because at the outset the only source of OH- is the water and this is 9 orders of magnitude less than the H+ from the acetic acid.
The formula can be adapted for the equilibrium point on titrating with 0.1M sodium hydroxide.
as follows
pH = 0.5pKw + 0.5pKa + 0.5logC
pH = 7 + 2.37 + (-0.65) = 8.72
where C is the acetic acid initial concentration.
Sorry, but - first of all - equation you derived:
[H^+] = \sqrt{[HA] K_a}
or
pH = \frac 1 2 pK_a + \frac 1 2 \log{[HA]}
is not a Henderson-Hasselbalch equation. Henderson-Hasselbalch equation is the one used for calculation of a buffer pH:
pH = pK_a + \log \frac {[A^-]}{[HA]}
See for example:
http://www.biology.arizona.edu/biochemistry/problem_sets/ph/HH.html
http://www.wiley.com/college/pratt/0471393878/student/review/acid_base/6_hh_equation.html
http://www.chemteam.info/AcidBase/HH-Equation.html
http://chemistry.about.com/od/acidsbase1/a/hendersonhasselbalch.htm
http://people.rit.edu/pac8612/webionex/website/html/ione89wu.html
http://www.lsbu.ac.uk/biology/biolchem/acids.html
and so on.
Now to the derivation:
As far as I know equation you listed has no name, and I am afraid there are several problems with your derivation and calculations.
Your final equation (bottom of the first page) uses [HA] which you used to denote equilibrium concentration of HA (undissociated acid). You can't use it to calculate pH, as you don't know it. What you usually know is the analytical concentration C
a and that's the information your final result should depend on. C
a should be introduced into the problem through the mass balance of the acid:
C_a = [HA] + [A^-]
You calculated correct pH value only because you entered
analytical concentration of the acid where your equation shows
equilibrium concentration of HA. This can be done after using another crucial approximation, that you have not mentioned - if the acid is not dissociated more than 5% we can assume:
[HA] \approx C_a
and your equation becomes
[H^+] = \sqrt{C_a K_a}
which is the final and correct form. Luckily for you 5% condition is met for 0.1M acetic acid solution, so pH=2.87 is a correct result, but for example for 10
-4M solution you would be off by 0.1 pH unit.
Compare
calculation of pH of weak acids (eq. 8.13), where this equation is not only derived, but also its applicability discussed.