Turion
- 145
- 2
Equation in pink:
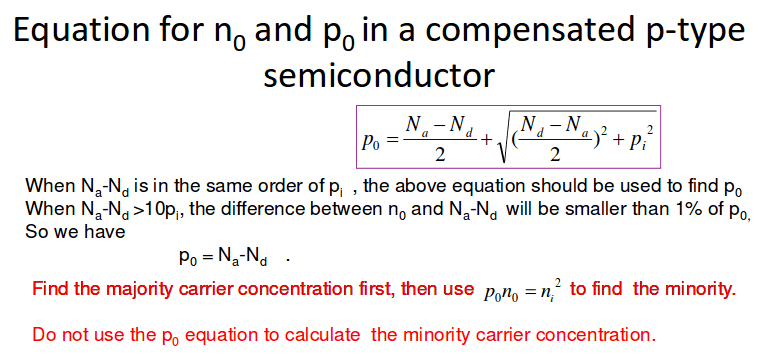
I was absent so I was going through the slides and I saw that equation. There isn't a derivation shown. Do you guys have any ideas?
I was absent so I was going through the slides and I saw that equation. There isn't a derivation shown. Do you guys have any ideas?
Last edited: