- #1
aiyoshi
- 1
- 0
An inventor proposes to make a heat engine using water/ice as the working substance inside a cylindrical piston and taking advantage of the fact that water expands as it freezes and can therefore lift a piston supporting some mass m. The engine process consists of four steps as shown in the schematic below.
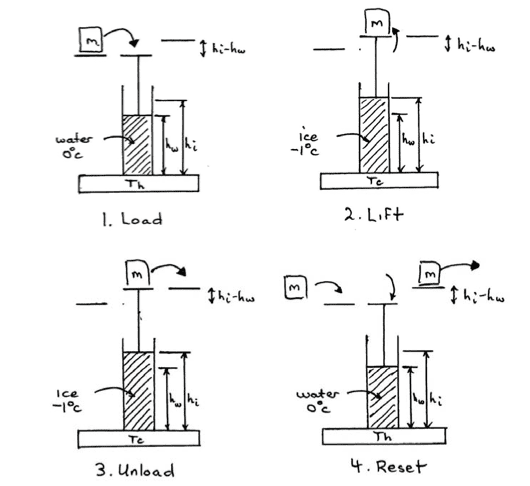
(i) Load: The weight to be lifted is placed on top of a piston over a cylinder of water held at a temperature of 1oC. The piston sits at height hw.
(ii) Lift: The system is then placed in thermal contact with a low temperature reservoir at −1oC until the water freezes into ice, lifting the weight to a height hi.
(iii) Unload: The weight is then removed at height hi while the ice remains frozen.
(iv) Reset: The ice is melted by putting it back in contact with the high-temperature reservoir at 1oC, returning the piston hw. Another mass is added to the piston and the cycle is ready to be repeated.
The inventor is pleased with this device because it can seemingly perform an unlimited amount of work (by lifting an unlimited mass m) while absorbing only a finite amount of heat each cycle.
Question
Assuming that the piston has a cross-sectional area of 10 cm2 and contains 50 cm3 of liquid H2O (i.e. homework = 5 cm), calculate:
(i) The work done by the piston in raising a mass of 10 g.
(ii) The mass required to stop the engine working (i.e., reduce the freezing point of the water to −1oC).
(i) Load: The weight to be lifted is placed on top of a piston over a cylinder of water held at a temperature of 1oC. The piston sits at height hw.
(ii) Lift: The system is then placed in thermal contact with a low temperature reservoir at −1oC until the water freezes into ice, lifting the weight to a height hi.
(iii) Unload: The weight is then removed at height hi while the ice remains frozen.
(iv) Reset: The ice is melted by putting it back in contact with the high-temperature reservoir at 1oC, returning the piston hw. Another mass is added to the piston and the cycle is ready to be repeated.
The inventor is pleased with this device because it can seemingly perform an unlimited amount of work (by lifting an unlimited mass m) while absorbing only a finite amount of heat each cycle.
Question
Assuming that the piston has a cross-sectional area of 10 cm2 and contains 50 cm3 of liquid H2O (i.e. homework = 5 cm), calculate:
(i) The work done by the piston in raising a mass of 10 g.
(ii) The mass required to stop the engine working (i.e., reduce the freezing point of the water to −1oC).