- #1
phee
- 36
- 0
Is this
1,3 -butene?
or just plain butene?
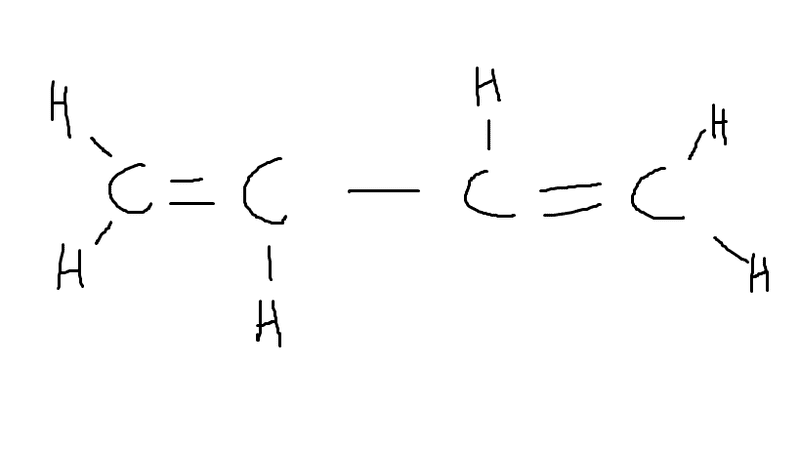
1,3 -butene?
or just plain butene?
phee said:So if there is two double bonds its di-"prefix"-ede ?
phee said:So if there is two double bonds its di-"prefix"-ede ?
gabriels-horn said:That is correct, 'ene' though, not 'ede'
Borek said:Somehow I fail to see "prefix" between di and ene.
1,3-butene and butene are both chemical compounds known as alkenes. However, they differ in their molecular structure. 1,3-butene has a double bond between the first and third carbon atoms, while butene has a double bond between the first and second carbon atoms.
Both 1,3-butene and butene are colorless gases at room temperature. However, 1,3-butene has a higher boiling point and melting point compared to butene due to its longer molecular chain.
1,3-butene is commonly used in the production of plastics, such as polyethylene and polypropylene. It is also used as a solvent and in the production of synthetic rubber. Butene is used in the production of gasoline and as a starting material in the production of other chemicals.
Both 1,3-butene and butene are flammable gases and should be handled with caution. In high concentrations, they can also cause irritation to the respiratory system. However, when used and stored properly, they do not pose significant hazards.
1,3-butene and butene are considered greenhouse gases and play a role in contributing to climate change. The production and use of these compounds also release other pollutants, such as carbon monoxide and particulate matter, which can harm the environment.