- #1
frostchaos123
- 17
- 0
Homework Statement
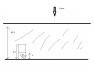
Referring to the attachment, a cylinder of 2.5m filled with air is submerged to a dept of 82.3m into the sea such that sea water in cylinder is now x m. Water at bottom is 277.15K.
The Attempt at a Solution
Using ideal gas law PV=nRT of air,
the answer gives (1atm + 82.3*rho*g) * (2.5 - x) * Area of cylinder = nR(277.15)
However i don't understand why the pressure of the gas is 1 atm + 82.3*rho*g. Since the pressure of gas is dependant on height of water, shouldn't it be 1 atm + (82.3-x) * rho*g instead?
Or another reasoning is shouldn't it be like pressure of air + pressure of water trapped in cylinder = pressure at the bottom of the sea?
Attachments
Last edited: