- #1
djsourabh
- 69
- 0
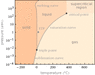
What is the exact scientific reason behind anamalous behavour of water?
Can the temperature range at which this happens be changed?
do any other substances also behave anamalously?
Can the temperature range at which this happens be changed?
do any other substances also behave anamalously?