physicsdude30
- 14
- 0
Note: This isn't intended to be a Global Warming thread but rather a Greenhouse Gases thread (they're not the same thing), so please let's not talk about Global Warming because I don't want it closed. I'm very interested on any feedback or thoughts anyone may have for my experiments.
Galileo would test things out for himself. We've all heard about greenhouse gases, but then I've heard some say they don't exist or that CO2 isn't one of them. So I thought it would be lots of fun to test this out for myself.
As you've probably noticed, during the daytime it's warmer than at night, so the Sun's rays warm the earth. You've probably noticed that the higher elevations aren't as warm, which is because there's "less atmosphere" up there. Also, just like some foods warm up better in the microwave than others, because of how the microwaves excite the molecules, scientists say sunlight warms up some gases like CO2 better than N2 or O2. So my train of thought is just like in the Medical Field where they test a new medicine by using an experiment and control group, it could be done with two glass jars the same with the difference being CO2 between them, then measure temperatures. This is what I've tried so far, so check it out and let me know what you think?
The two glass jars I used

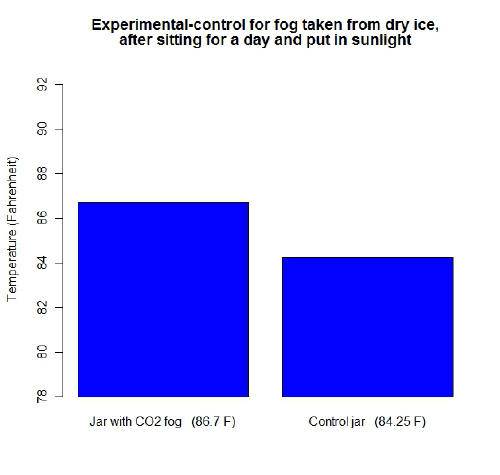
First I decided to use CO2 gas from dry ice. I put some ice in a separate glass of water and let it fog over.

I let the fog catch in the experimental jar. Both the jars were sealed off, let sit overnight to allow CO2 to dissipate, and then temperatures taken during the sunlight the next day. This is what I got. Need some feedback?:
Next I thought it would be interesting to try match stick smoke. I rinsed out both jars and dried them, then held some burning match sticks above the upside down experimental jar. Although more than "CO2 gas" was in the jar, I thought it was appropriate enough of a test since one of the ways among many it gets into the atmosphere is "burning substances". I let both jars sit over night so they could have the chance to be the same temperature. The next day when both of them were in sunlight this is what I got:
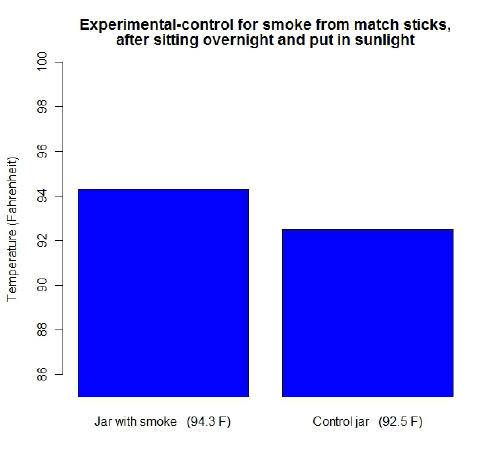
Why I thought about testing match stick smoke:

Just looking for feedback on these experiments!
Galileo would test things out for himself. We've all heard about greenhouse gases, but then I've heard some say they don't exist or that CO2 isn't one of them. So I thought it would be lots of fun to test this out for myself.
As you've probably noticed, during the daytime it's warmer than at night, so the Sun's rays warm the earth. You've probably noticed that the higher elevations aren't as warm, which is because there's "less atmosphere" up there. Also, just like some foods warm up better in the microwave than others, because of how the microwaves excite the molecules, scientists say sunlight warms up some gases like CO2 better than N2 or O2. So my train of thought is just like in the Medical Field where they test a new medicine by using an experiment and control group, it could be done with two glass jars the same with the difference being CO2 between them, then measure temperatures. This is what I've tried so far, so check it out and let me know what you think?
The two glass jars I used
First I decided to use CO2 gas from dry ice. I put some ice in a separate glass of water and let it fog over.
I let the fog catch in the experimental jar. Both the jars were sealed off, let sit overnight to allow CO2 to dissipate, and then temperatures taken during the sunlight the next day. This is what I got. Need some feedback?:
Next I thought it would be interesting to try match stick smoke. I rinsed out both jars and dried them, then held some burning match sticks above the upside down experimental jar. Although more than "CO2 gas" was in the jar, I thought it was appropriate enough of a test since one of the ways among many it gets into the atmosphere is "burning substances". I let both jars sit over night so they could have the chance to be the same temperature. The next day when both of them were in sunlight this is what I got:
Why I thought about testing match stick smoke:
Just looking for feedback on these experiments!