You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Sublimation Definition and 31 Threads
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface.
At normal pressures, most chemical compounds and elements possess three different states at different temperatures. In these cases, the transition from the solid to the gaseous state requires an intermediate liquid state. The pressure referred to is the partial pressure of the substance, not the total (e.g. atmospheric) pressure of the entire system. So, all solids that possess an appreciable vapour pressure at a certain temperature usually can sublime in air (e.g. water ice just below 0 °C). For some substances, such as carbon and arsenic, sublimation is much easier than evaporation from the melt, because the pressure of their triple point is very high, and it is difficult to obtain them as liquids.
The term sublimation refers to a physical change of state and is not used to describe the transformation of a solid to a gas in a chemical reaction. For example, the dissociation on heating of solid ammonium chloride into hydrogen chloride and ammonia is not sublimation but a chemical reaction. Similarly the combustion of candles, containing paraffin wax, to carbon dioxide and water vapor is not sublimation but a chemical reaction with oxygen.
Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor phase. Since the process requires additional energy, it is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated by adding the enthalpy of fusion and the enthalpy of vaporization.
View More On Wikipedia.org
At normal pressures, most chemical compounds and elements possess three different states at different temperatures. In these cases, the transition from the solid to the gaseous state requires an intermediate liquid state. The pressure referred to is the partial pressure of the substance, not the total (e.g. atmospheric) pressure of the entire system. So, all solids that possess an appreciable vapour pressure at a certain temperature usually can sublime in air (e.g. water ice just below 0 °C). For some substances, such as carbon and arsenic, sublimation is much easier than evaporation from the melt, because the pressure of their triple point is very high, and it is difficult to obtain them as liquids.
The term sublimation refers to a physical change of state and is not used to describe the transformation of a solid to a gas in a chemical reaction. For example, the dissociation on heating of solid ammonium chloride into hydrogen chloride and ammonia is not sublimation but a chemical reaction. Similarly the combustion of candles, containing paraffin wax, to carbon dioxide and water vapor is not sublimation but a chemical reaction with oxygen.
Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor phase. Since the process requires additional energy, it is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated by adding the enthalpy of fusion and the enthalpy of vaporization.
View More On Wikipedia.org
-
A
I How Long Does Water Ice Take to Sublimate in Space?
How long does it take water ice H20 in space in our solar system to sublimate, say a basic ice cube? It starts as a solid cube at the temperature of whatever space is above Earth and then completely turns to vapor. Just looking for ballpark situation here. Does anyone know of a table or place...- Albertgauss
- Thread
-
- Tags
- Ice Space Sublimation Time
- Replies: 7
- Forum: Astronomy and Astrophysics
-
H
I Comet Sublimation: Why Does Halley's Comet Still Exist?
Having watched a dropped cube of ice sublime to nothing at the bottom of the freezer of my refrigerator in my kitchen over the course of a week, I would like to know why comets don't do this. Presumably the tail is the product of sublimation, but how long can that go on? How can Halley's Comet...- hexanol
- Thread
-
- Tags
- Comet Sublimation
- Replies: 6
- Forum: Astronomy and Astrophysics
-

Is the Triple Point of Water Affected by Atmospheric Pressure?
Hello, I have a little difficulty understanding how sublimation works, from what I have understood so far, the sublimation of ice for example occurs below its triple point (at a certain pressure and temperature but not atmospheric pressure but at a certain partial pressure). Does this mean that...- Sphere
- Thread
-
- Tags
- Sublimation Work
- Replies: 6
- Forum: Chemistry
-
M
Can coatings prevent sublimation?
Question for materials scientists: All solids evaporate (or sublimate) over some time, even steel. Could the sublimation of steel over time be prevented or slowed with a coating? EDIT: Do diamonds themselves sublimate in a vacuum?- Mgt3
- Thread
-
- Tags
- Sublimation
- Replies: 3
- Forum: Materials and Chemical Engineering
-
D
Relation between enthelpy of sublimation and rate of sublimation?
Hello, I am running experiments where materials are heated at high temperatures during tens of hours under high-vacuum conditions. Since what I am investigating lies in the first hundreds of nanometers of the materials, I must take into account (and anticipate) surface sublimation. Therefore I...- DenisH
- Thread
-
- Tags
- Rate Relation Sublimation
- Replies: 1
- Forum: Materials and Chemical Engineering
-

Clausius-Clapeyron equation to estimate sublimation pressure of water
Before this question, the questions were about the Clapeyron equation, and how to estimate ##\Delta s##. I'm completely put off by this question however, and don't know where to start. I've found that the triple point of water is at ##0.01°C##, and there is indeed data in the table for...- WhiteWolf98
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-

Chemistry: Heat of Sublimation and Hydrogem Bonds Problem
Homework Statement Using the heats of fusion and vaporization for water, calculate the change in enthalpy for the sublimation of water: H2O(s) --> H2O(g Using the delta H value given in Exercise 24 and the number of hydrogen bonds formed to each water molecule, estimate what portion of the...- komarxian
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
S
No sublimation for human body in vacuum? [Thermodynamics]
In my Thermodynamics course, we recently learned about the saturation pressure and saturation temperature of different substances -mainly water. As you know, the saturation temperature at which a substance begins to boil is specific to a fixed saturation pressure. Water only boils at 100...- Satonam
- Thread
- Replies: 1
- Forum: Thermodynamics
-
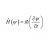
Sublimation: invariant heat or internal energy?
Homework Statement Below, two experiments (1 and 2) are described, in which the same quantity of solid carbon dioxide is completely sublimated, at 25ºC: The process is carried out in a hermetically sealed container, non-deformable with rigid walls; The process is carried out in a cilinder...- Vitor Pimenta
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
E
I Is there a boiling analog to sublimation?
Liquid water sitting can evaporate without a problem, given that the vapor pressure surrounding it is less than the temperature dependent saturated vapor pressure. Similarly, an ice cube can also evaporate without becoming liquid under these same conditions. However, an extreme form of...- Electric to be
- Thread
-
- Tags
- Analog Boiling Sublimation
- Replies: 1
- Forum: Other Physics Topics
-

Evaporating water using heat and reducing vapour pressure
I want to reduce the amount of water present in the digestate coming out of my Anaerobic Digester. The traditional method would be to boil it until I've removed as much off as I want. However, this is expensive from an energy consumption point of view. There's a great video on Youtube showing a...- jmarkwalker
- Thread
- Replies: 8
- Forum: General Engineering
-

Sublimation of Graphite: Understanding the Process and Its Significance
Homework Statement Multiple choice:[/B] In which of the following processes are covalent bonds broken? a) solid sodium chlroide melts b) bronze melts c) sucrose dissolves in water d) solid carbon graphite sublimes e) solid carbon dioxide sublimes Homework Equations none The Attempt at a...- RoboNerd
- Thread
-
- Tags
- bonds change graphite sublimation
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
J
CO2 sublimation in a closed container
A coworker posed a thought experiment; if you sealed a sample of frozen CO2 in an uncompressable container of identical volume and allowed it to warm to room temperature, how could it melt/evaporate, since there would be no room for the gas to expand My guess is that it would sublimate to a...- Jonhorde
- Thread
-
- Tags
- Closed Co2 Container Sublimation
- Replies: 3
- Forum: Other Physics Topics
-

Which are the elements which direcly sublimes
I know that Arsenic sublimes directly when heated. So I want to know which other elements like this sublimates on heating throughout the periodic table. I also know dry ice. thanks- Prins
- Thread
-
- Tags
- Elements Sublimation
- Replies: 3
- Forum: Atomic and Condensed Matter
-
N
Dry ice sublimation, heat balance
Homework Statement The problem is this: what is the optimum amount of dry ice inside the Styrofoam pack, if we want to received a frozen dough (temp.-18) after 3 days of transportation in temperature 20 C ? My calculations and data placed in the attached excel file, but I'm not sure if they...- nooldor
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
Q
Sublimation temperature of water in vacuum = 150K ?
I am trying to find references for the the Wikipedia article Frost line (astrophysics) I am having a hard time finding a reference for the "Sublimation temperature of water in vacuum" that is used for calculation of the current snow line. E.g.: I have found 150K in several places, without any...- Qshadow
- Thread
- Replies: 18
- Forum: Astronomy and Astrophysics
-
T
How Do You Calculate the Changing Mass of a Sublimating Solid?
Hi all, I am forced to step outside of my field to investigate a problem that might root in sublimation of solids. I am trying to calculate the mass (after a certain time) of a material that we know is sublimating at , say .000123 g/(cm^2 hr). Is it as simple as multiplying the rate by the...- tempneff
- Thread
-
- Tags
- Rate Solids Sublimation
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
H
Understanding Freeze-Drying: IR Radiations, Sublimation & Phase Transitions
Well! the material to be freeze-dried is irradiated with IR radiations before, why?? Also I am confused as to how can ice sublime at atmospheric pressure?? Isn't the triple point of water much lower? is the vacuum around the material created for this reason? ( to lower the pressure I mean) Also...- Himanshu_6174
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
X
Calculating sublimation temperature
Homework Statement The standard enthalpy of sublimation of dry ice (solid carbon dioxide) is 6.03 kJ/mol. The triple point of CO2 is at 5.1 atm, -59.7 °C. Calculate the normal sublimation temperature of CO2 at 1.00 atm pressure. Homework Equations Delta G= Delta H-T(delta S)...- xdrgnh
- Thread
-
- Tags
- Sublimation Temperature
- Replies: 10
- Forum: Biology and Chemistry Homework Help
-
Q
Calculating Final Pressure in a Sublimation and Pressure Homework Problem
Homework Statement When fluorine and solid iodine are heated at 550. K, the iodine completely sublimes and gaseous iodine heptafluoride forms. If 350. torr of fluorine gas and 2.50 g of solid iodine are put into a 2.50 L container at 250. K and the container is heated to 550. K, what is the...- Qube
- Thread
-
- Tags
- Pressure Sublimation
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
C
Chromium/Vanadiam Sublimation or Melting
I have a pretty stupid question that I hope someone can answer. I'm trying to deposit chromium and vanadium in UHV, and I need to determine whether each of the materials will "melt" or "sublimate" so that I know the volume to fill my crucible. My impression is that these materials will...- citw
- Thread
-
- Tags
- Melting Sublimation
- Replies: 1
- Forum: Atomic and Condensed Matter
-
M
Sublimation of MP & VP: Separating Compounds
I am wondering if I can separate these two compounds: Y: M.P.=170 degrees C - Vapor pressure at its MP = 0.05 torr Z: M.P.=170 degrees C - Vapor pressure at its MP = 540 torr I think having a huge difference in vapor pressures is good. I am kind of confused about this process.- morgan8222
- Thread
-
- Tags
- Sublimation
- Replies: 1
- Forum: Chemistry
-
F
Calculate sublimation rate from pressure rise
Hi, I did a pressure rise test on a chamber, where I closed the isolation valve and measured the increase in pressure over 30 seconds. I want to use this information to calcualte the rate of accumulation of vapour in the chamber. What I'm looking for is the sublimation rate during freeze...- flysa01
- Thread
-
- Tags
- Pressure Rate Rise Sublimation
- Replies: 2
- Forum: Materials and Chemical Engineering
-

Sublimation of Ice at Atmospheric Pressure
Homework Statement How can ice sublimate at atmospheric pressure? Looking at the p-T diagram for water, the sublimation curve does not extend above a tiny fraction of atmospheric (triple point of water, p = 4.58mm Hg). Yet my ice box tells a different story. Homework Equations...- rude man
- Thread
-
- Tags
- Ice Sublimation
- Replies: 18
- Forum: Advanced Physics Homework Help
-
I
Sublimation of Dry Ice in a Closed System
Hello, Please forgive my ignorance, although bright, I was a lousy student, & never took physics in school. I find it frustrating when relatives & friends are uncertain as to how to respond to questions like those below, so your educated reply would therefore be all the more appreciated...- Inquirer18
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
L
Is Ablimation the Same as Sublimation?
Hi, Does anyone know what ablimation is? Is it the same as sublimation? Regards- lesy1
- Thread
-
- Tags
- Sublimation
- Replies: 2
- Forum: Other Physics Topics
-
S
Caffeine sublimation and melting point
Why caffeine has 178 oC as point of sublimation but 237 oC as melting point? Is it about the pressure? If so, then what is the preesure that makes 237 as melting point instead of sublimation at 178 oC? Cuz the science websites always just said 237 is the melting point, but didn't mention the...- slft
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
B
How Much Ice Melts to Lower Water Temperature from 24°C to 5°C?
Homework Statement How much ice (in grams) would have to melt to lower the temperature of 351 ml of water from 24 deg C to 5 deg C? (Assume the density of water is 1.0g/ml .) Homework Equations q=mc(deltaT) The Attempt at a Solution m=351 g c=4.18J/g*C deltaT=19deg C...- blueskadoo42
- Thread
-
- Tags
- Fusion Sublimation
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
Z
Understanding Sublimation: Principle and Process Explained
On what principle does a substance sublime? I mean , does it work towards thermal equillibrium and hence the gain in heat raises the Kinetic energy to hence change the state to vapour? But then sublime substances still sublime when equillibrium has been attained.- zd1899
- Thread
-
- Tags
- Sublimation
- Replies: 3
- Forum: Atomic and Condensed Matter
-
K
Why substances can undergo sublimation and turn into gas?
Why is is that certain substances, (example: solid carbon dioxide) can undergo sublimation and turn into gas without undergoing liquid form when heated? What is the general characteristics that enable certain substances to undergo sublimation? Is it possible to have a substance that can...- kfa
- Thread
-
- Tags
- Gas Sublimation
- Replies: 2
- Forum: Other Physics Topics
-
T
Why Do Solids Sublime into Gas?
Any one knows the reason for the Sublimation( solid to gas). I think the attraction between the air molecules and the weaker molecular stuctured solid molecules. its and adhesion effect. the molecules in surface of the solid is being attracted by the air molecules ( such like polar molecules NO2...- THANApHD
- Thread
-
- Tags
- Sublimation
- Replies: 3
- Forum: Chemistry