- #1
patric44
- 296
- 39
- Homework Statement
- iam having a little problem related to solving the theta part for the hydrogen atom
- Relevant Equations
- problem with the theta part of the SE and associated Legendre function
hi guys
i am having a little problem concerning the theta part of TISE :
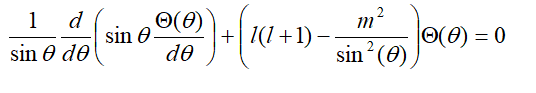
its clearly that its very similer to the associated Legendre function :
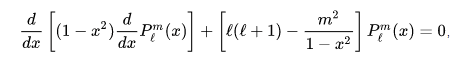
how iam going to change 1/sinθ ... to (1-x^2) in which x = cosθ
i tried many identities but i am stuck here .
any help on that ?
i am having a little problem concerning the theta part of TISE :
its clearly that its very similer to the associated Legendre function :
how iam going to change 1/sinθ ... to (1-x^2) in which x = cosθ
i tried many identities but i am stuck here .
any help on that ?