- #1
EEristavi
- 108
- 5
Hello,
I read article how to check battery condition appropriately (e.g. car battery).
(Link: https://www.allaboutcircuits.com/textbook/direct-current/chpt-11/battery-ratings/ )
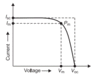
It's written that: in order to get better results, one must check under load.
I'm not EE - I study physics and this fact is not clear for me:
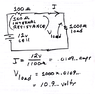
When you see battery, its written 12V (for example), so it is voltage source.
From previous assumption (its fixed voltage source): potential difference shouldn't be dependent on resistance. But as a fact it does - if we see voltage without load it shows different value compared to voltage when we have a load.
So, if its not fixed voltage source, than why giving voltage and amp-hour and not power
I read article how to check battery condition appropriately (e.g. car battery).
(Link: https://www.allaboutcircuits.com/textbook/direct-current/chpt-11/battery-ratings/ )
It's written that: in order to get better results, one must check under load.
I'm not EE - I study physics and this fact is not clear for me:
When you see battery, its written 12V (for example), so it is voltage source.
From previous assumption (its fixed voltage source): potential difference shouldn't be dependent on resistance. But as a fact it does - if we see voltage without load it shows different value compared to voltage when we have a load.
So, if its not fixed voltage source, than why giving voltage and amp-hour and not power