- #1
fab13
- 312
- 6
Thread moved from the technical forums, so no Homework Template is shown
as homework, I have to do the following exercise:
Bremstrahlung emission :
We are interested in ionized plasma of density ##n##, at temperature ##T## with ions of charge number ##Z##.
1. Explain the physical origin of bremstrahlung radiation
2. Represent the expected spectra (shape and remarkable/points values)
3. Explain how the total power emitted depend of properties of plasma.
I would like to make things clearer :
1) For question 1), I saw that the bremsstrahlung radiation corresponds to the radiation emitted by a particle in deceleration, hence the name of radiation "braking".
What if the particle is accelerating: are we also talking about radiation bremsstrahlung?
Indeed, if we consider that the particle has a centripetal acceleration, as in the case of displacement in a magnetic field, we speak rather of synchrotron radiation, is that correct?
2) For question 2), we are asked to qualitatively represent the typical form of the bremsstrahlung spectra (with its points and remarkable values): someone could tell me what is this expected spectra and the values / points to to retain for this spectra (a typical spectra) ?
3) Finally, the question n ° 3) concerns the expression of the total power emitted per unit of volume, according to what I have on Wiki, this quantity is equal to :
##P_{\mathrm {Br} }[{\textrm {W/m}}^{3}]=\left[{n_{e} \over 7.69\times 10^{18}{\textrm {m}}^{-3}}\right]^{2}T_{e}[{\textrm {eV}}]^{1/2}Z_{\mathrm {eff}##
Here the formula :
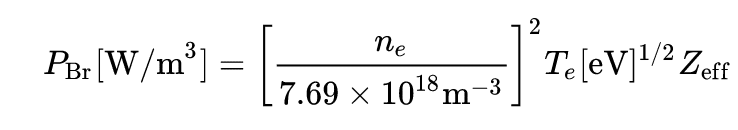
The total power would then depend of : the temperature ##T## of the plasma, its density ##n## and the number of charge ##Z## of the ions of the plasma: is this correct?
Thanks for your help
Bremstrahlung emission :
We are interested in ionized plasma of density ##n##, at temperature ##T## with ions of charge number ##Z##.
1. Explain the physical origin of bremstrahlung radiation
2. Represent the expected spectra (shape and remarkable/points values)
3. Explain how the total power emitted depend of properties of plasma.
I would like to make things clearer :
1) For question 1), I saw that the bremsstrahlung radiation corresponds to the radiation emitted by a particle in deceleration, hence the name of radiation "braking".
What if the particle is accelerating: are we also talking about radiation bremsstrahlung?
Indeed, if we consider that the particle has a centripetal acceleration, as in the case of displacement in a magnetic field, we speak rather of synchrotron radiation, is that correct?
2) For question 2), we are asked to qualitatively represent the typical form of the bremsstrahlung spectra (with its points and remarkable values): someone could tell me what is this expected spectra and the values / points to to retain for this spectra (a typical spectra) ?
3) Finally, the question n ° 3) concerns the expression of the total power emitted per unit of volume, according to what I have on Wiki, this quantity is equal to :
##P_{\mathrm {Br} }[{\textrm {W/m}}^{3}]=\left[{n_{e} \over 7.69\times 10^{18}{\textrm {m}}^{-3}}\right]^{2}T_{e}[{\textrm {eV}}]^{1/2}Z_{\mathrm {eff}##
Here the formula :
The total power would then depend of : the temperature ##T## of the plasma, its density ##n## and the number of charge ##Z## of the ions of the plasma: is this correct?
Thanks for your help