- #1
WMDhamnekar
MHB
- 376
- 28
Hello,
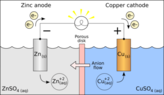
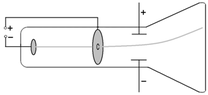
In cathode ray discharge tube,negative electrode is cathode and positive electrode is anode.
But in case of direct current circuit, it is the positively charged terminal of a voltaic cell or storage battery that supplies current and anode is the negatively charged terminal of a voltaic cell or storage battery that supplies current.
Why?
In cathode ray discharge tube,negative electrode is cathode and positive electrode is anode.
But in case of direct current circuit, it is the positively charged terminal of a voltaic cell or storage battery that supplies current and anode is the negatively charged terminal of a voltaic cell or storage battery that supplies current.
Why?