- #1
miniradman
- 196
- 0
Homework Statement
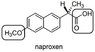
determine and explain its chirality using the CIP rule
*see attached image*
Homework Equations
CIP rules
The Attempt at a Solution
Hello
I've determined that the stereocenter of this molecule is at the carbon where the carboxyl group meets the two benzene rings.
I've done step one and rotated the stereocenter such that the hydrogen is facing back. However when deciding the priorities of the three constituents on the stereocenter I get a little confused.
When going down the chain, towards the aromatic rings that I'll encounter a double bond. I tried reading how these fit in with the CIP rules but I find them to be quite ambiguous.
And with the carboxyl group, when going down the chain, which direction do I have to go? Down the hydroxyl group on the double bonded oxygen? I
Thanks
- miniradman