- #1
riseofphoenix
- 295
- 2
Complcated Chemistry Reaction - impossible??
Help :(
My notes say:
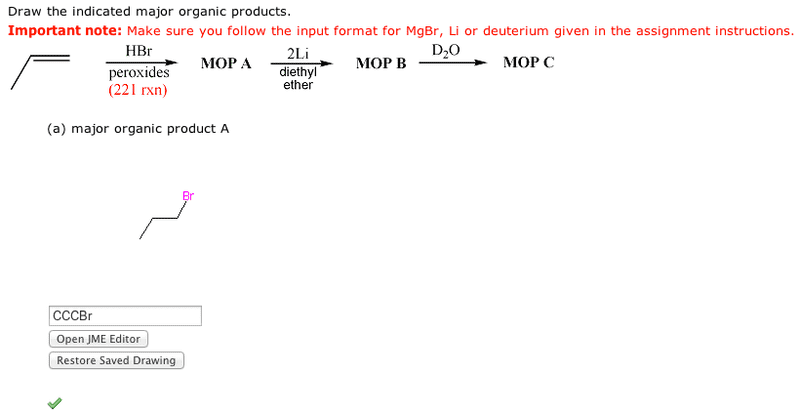
Step 1 is a 221 reaction. If peroxides are used in your version, then the anti-Markovnikov bromide is formed. If your version had only HBr, the the Markovnikov bromide is formeed.
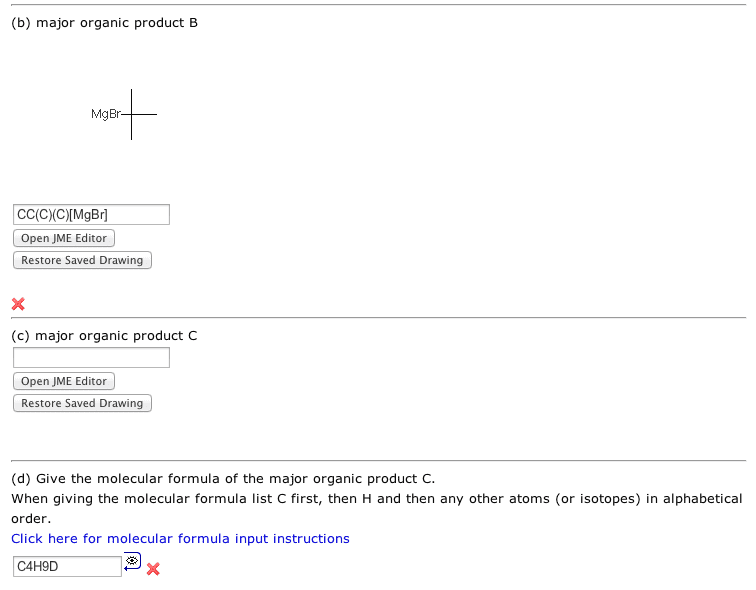
Step 2 creates organolithium or Grignard reagent (depending on your version).
Steps 3 is a strong acid (D2O) - strong base (organometallic carbon) reaction which gives a deuterated alkane.
-----
I managed to get A right but I can't get B right :(
Help :(
My notes say:
Step 1 is a 221 reaction. If peroxides are used in your version, then the anti-Markovnikov bromide is formed. If your version had only HBr, the the Markovnikov bromide is formeed.
Step 2 creates organolithium or Grignard reagent (depending on your version).
Steps 3 is a strong acid (D2O) - strong base (organometallic carbon) reaction which gives a deuterated alkane.
-----
I managed to get A right but I can't get B right :(