- #1
tade
- 702
- 24
Forgive me for this stupid question, but how do I convert between
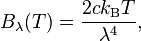
and
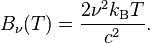
I tried c = νλ but that doesn't work. This is the Rayleigh Jeans Law by the way.
and
I tried c = νλ but that doesn't work. This is the Rayleigh Jeans Law by the way.
Last edited: