- #1
randomgamernerd
- 139
- 4
Homework Statement
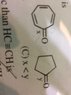
: [/B]The correct relation is:A. x=y
B. x>y
C. x<y
D. None.
Please refer to picture attached.
Homework Equations
: [/B]As stability increases, bond length decreases. When a Π bond is delocalised i.e it takes part in resonance, stability increases.The Attempt at a Solution
I think x takes part in resonance.
Hence x is more stable than y.
Thus x<y i.e C is correct option.