- #1
Physics345
- 250
- 23
Homework Statement
Experimental evidence suggests that the nitrogen atom in ammonia, NH3, has four identical orbitals in the shape of a pyramid or tetrahedron.
a) Draw an energy level diagram to show the formation of these hybrid orbitals.
(hint: No electron promotion is required)
b) Name the type of Hybrid orbitals found in NH3. Of the four hybrid orbitals on the N atom, how many will take part in bonding?
c)Draw for yourself the energy – level diagram showing the hybrid orbitals formed in the C atom when it bonds. Now look at those hybrid orbitals and those of the N atom, and describe how the bonding with a N atom will di²er with the bonding that occurs with a C atom, even though both atoms have four hybrid orbitals oriented in a tetrahedral shape
Homework Equations
None
The Attempt at a Solution
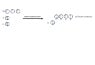
a)

b) sp^3 Is the hybrid orbital found in NH_3 in this case Nitrogen wants to complete its valence shell becoming like the noble gas Neon, thus it takes 3 electrons from each 1s orbital from the three hydrogen atoms and completes its 2p orbital forming a complete shell and a sp^3 hybridization. Therefore, it uses three hybrid orbitals.
c) The bonding with the nitrogen atom is different compared to the carbon atom. The carbon atom has 4 incomplete orbitals and only 2 electrons in its 2p orbitals while the third 2p orbital remains empty, while the nitrogen atom has 4 incomplete orbitals as well but the difference is that it has 3 electrons in each of its 2p orbitals.
I was just wondering if I did these correctly. Thanks in advance.