- #1
Special One
- 32
- 1
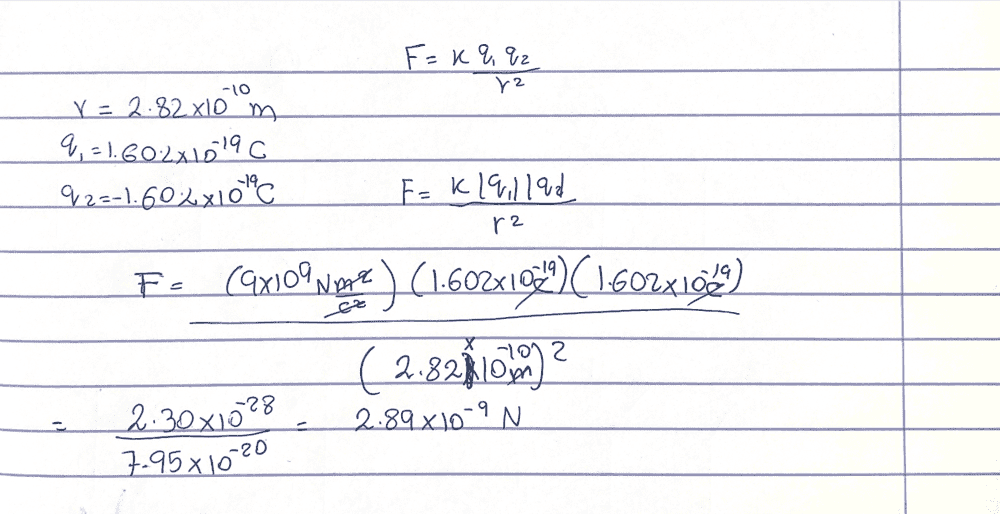
- Homework Statement
- Force
- Relevant Equations
- Coulomb's Law
In a salt crystal, the distance between adjacent sodium and chloride ions is 2.82×10^−10m. What is the force of attraction between the two singly charged ions?
Last edited by a moderator: