- #1
upender singh
- 14
- 0
Hi,
I am trying to model a counter flow diffusion flame using ansys fluent with fuel/oxidiser as methane/air.
The problem is that the flame temperature(800 K) is much lower than what it should be(>1600 K).
I am using Non premixed combustion module with steady diffusion flamelet and adiabatic energy treatment since combustion is instantaneous process. I am using a standard 30 species chemkin reaction mechanism from standard mechanisms library.
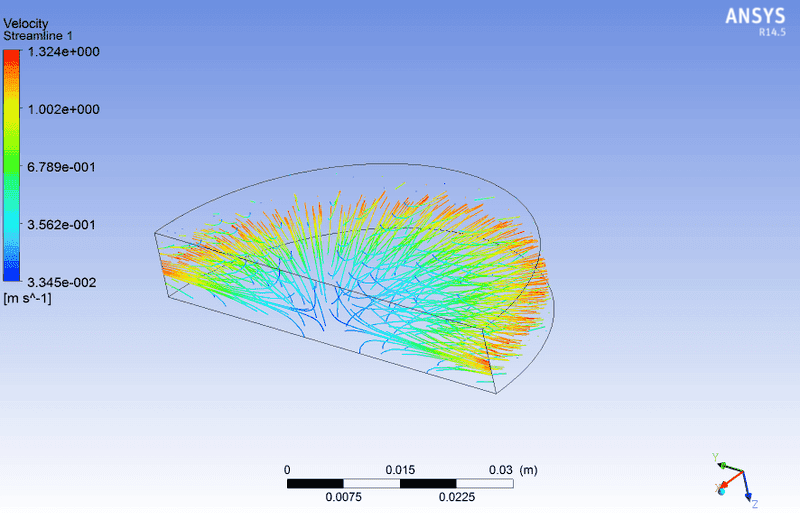
My PDF table seems OK as The plots show a max temperature of 1800 K at a mean mixture fraction value of 0.1. The geometry is a simple cylinder with two inlets in the axial direction and outlet in radial direction(image).
The Dia of two inlets is 60mm and the gap=10mm.
Can anybody explain why am I not getting the desired temperature? I would be happy to provide any further information if required.
I am trying to model a counter flow diffusion flame using ansys fluent with fuel/oxidiser as methane/air.
The problem is that the flame temperature(800 K) is much lower than what it should be(>1600 K).
I am using Non premixed combustion module with steady diffusion flamelet and adiabatic energy treatment since combustion is instantaneous process. I am using a standard 30 species chemkin reaction mechanism from standard mechanisms library.
My PDF table seems OK as The plots show a max temperature of 1800 K at a mean mixture fraction value of 0.1. The geometry is a simple cylinder with two inlets in the axial direction and outlet in radial direction(image).
The Dia of two inlets is 60mm and the gap=10mm.
Can anybody explain why am I not getting the desired temperature? I would be happy to provide any further information if required.