- #1
Beer-monster
- 296
- 0
Homework Statement
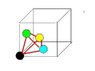
I'd like to work out what the largest sphere I could fit in the tetrahedral interstitial site of a cubic unit cell. Preferably I believe I need an expression in terms of the lattice constant a.
Homework Equations
The Attempt at a Solution
The tetrahedral site is at the centre of a 'minicube' defined by dividing each of the major lattice planes of the unit cell in half (resulting in 8 such 'minicubes' with edge length a/2).
Using this fact I thought I could work out the free space available by taking the total volume of the tetrahedron and subtracting the volume of the sphere fractions contained within the tetrahedron. Then working out the size of a sphere corresponding to the that free space.
However, this is proving rather messy and complicated (mainly due to working out what fraction of the spheres are contained in the tetrahedron). Also, I think I'm looking for a general solution and my method would differ if it was fcc or bcc cubes.
Could anyone point me in a better direction?
Thanks