- #1
SpartanG345
- 70
- 1
I am having a little trouble understanding de Broglies equation and wavelength and it relation ship with energy
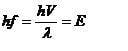
by E=hf and debroglies equation the above equation will get the energy of the particle
i did an example and i found this energy does not = the Kinetic energy of the particle, infact it was smaller
why is this
I thought E = hf only applies to zero mass particles?
by E=hf and debroglies equation the above equation will get the energy of the particle
i did an example and i found this energy does not = the Kinetic energy of the particle, infact it was smaller
why is this
I thought E = hf only applies to zero mass particles?